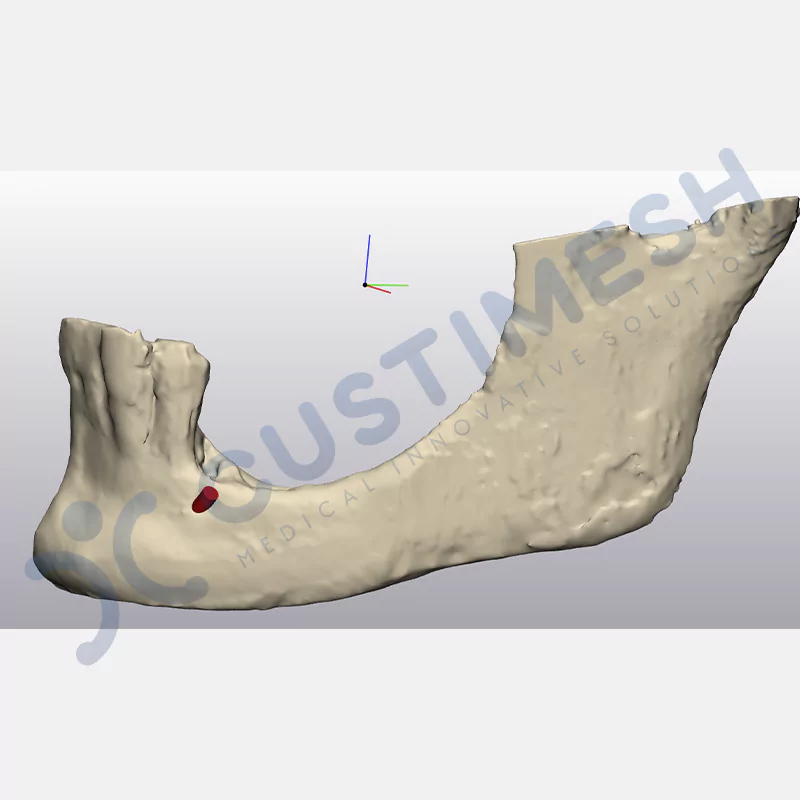
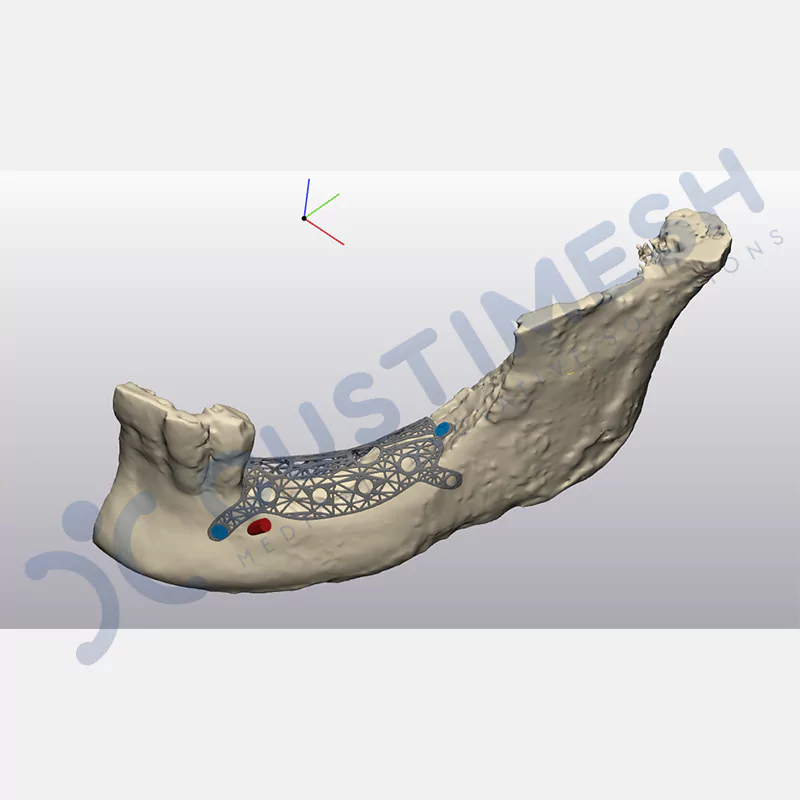
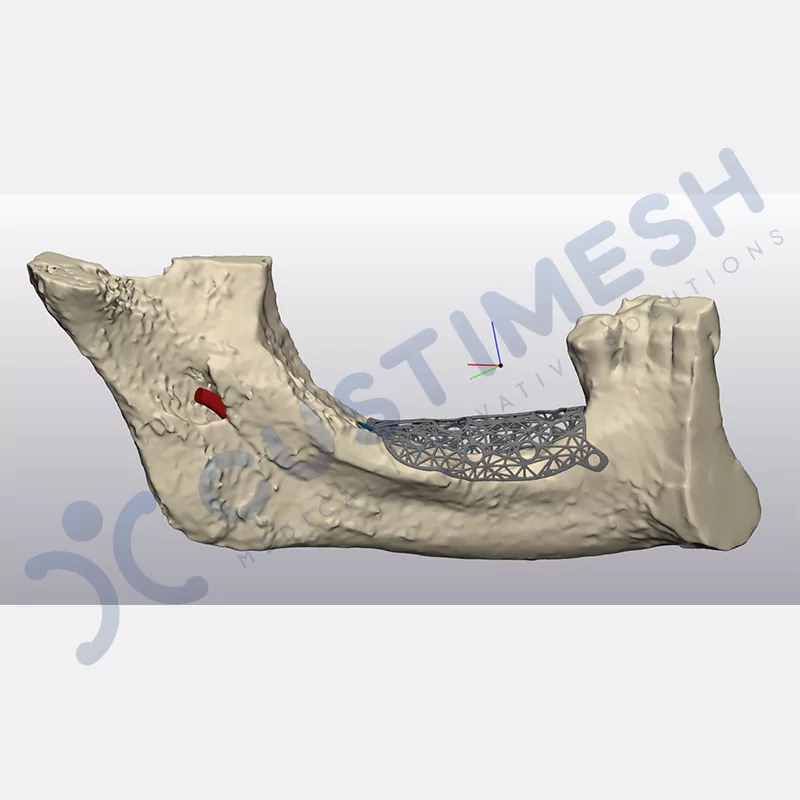
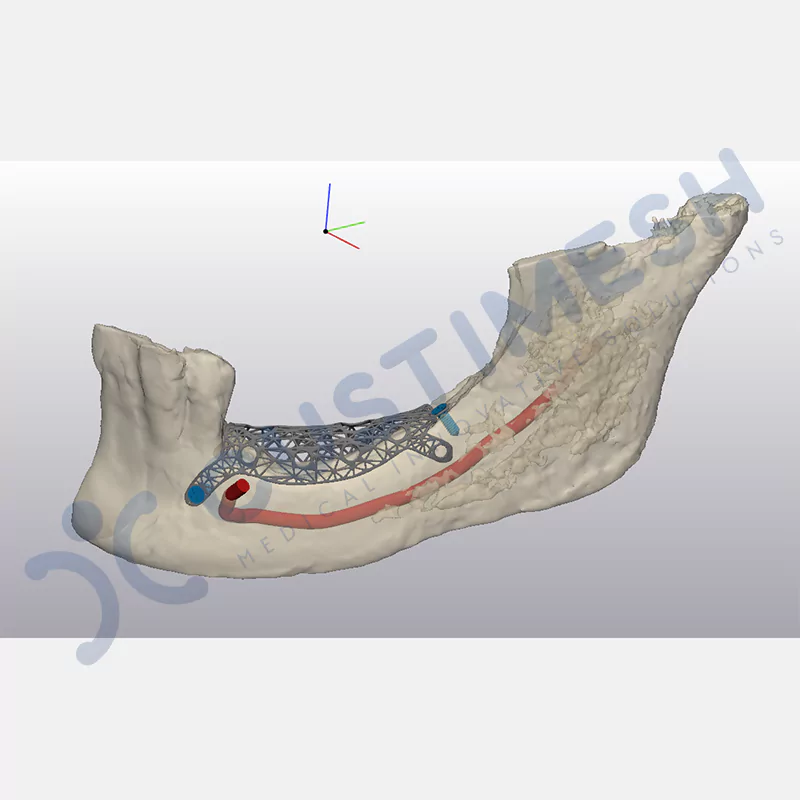
Innovative medical solution for bone regeneration
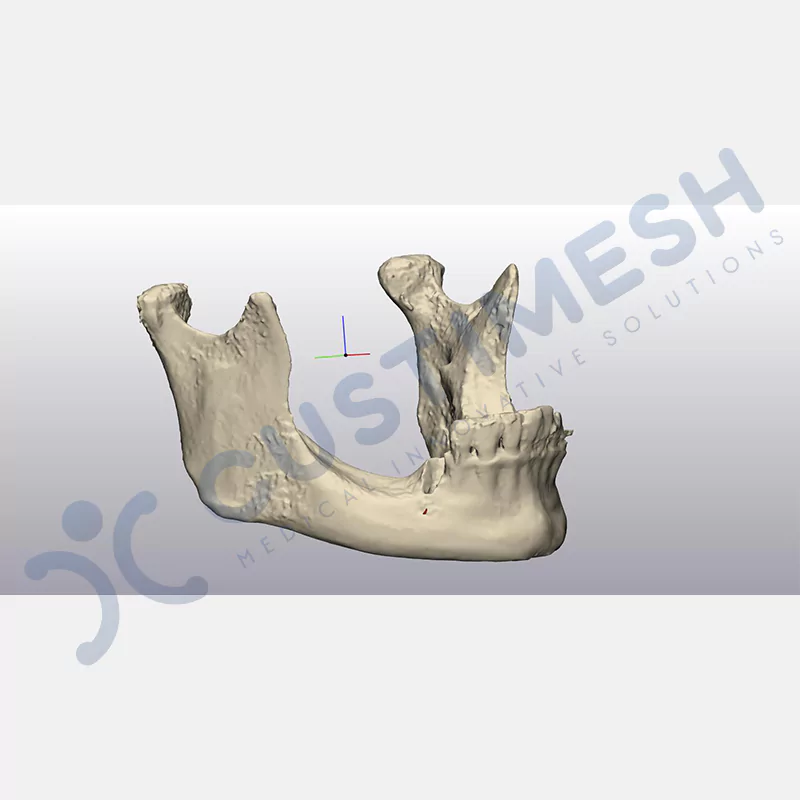
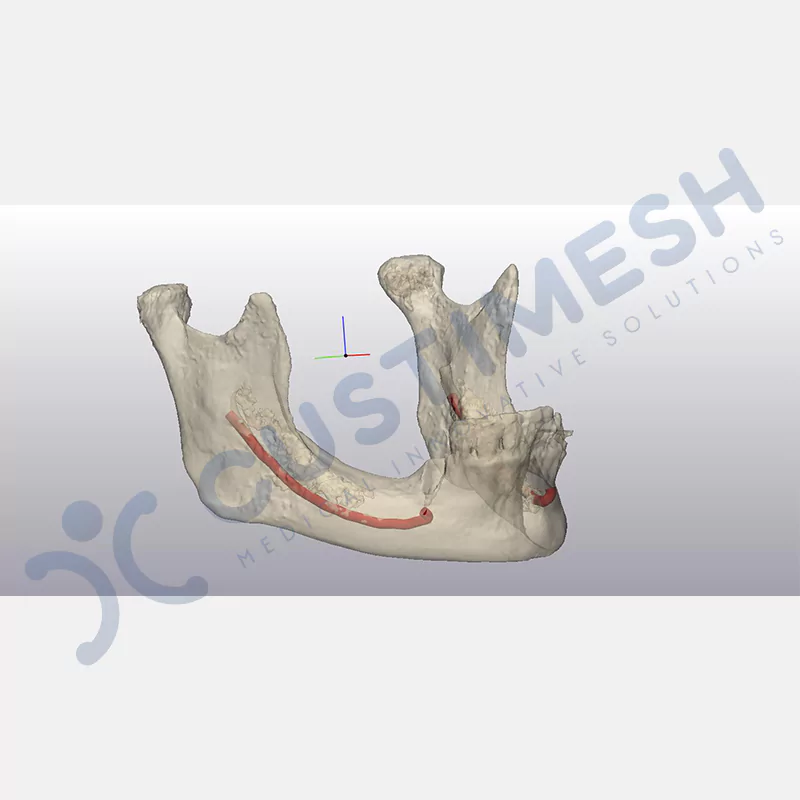
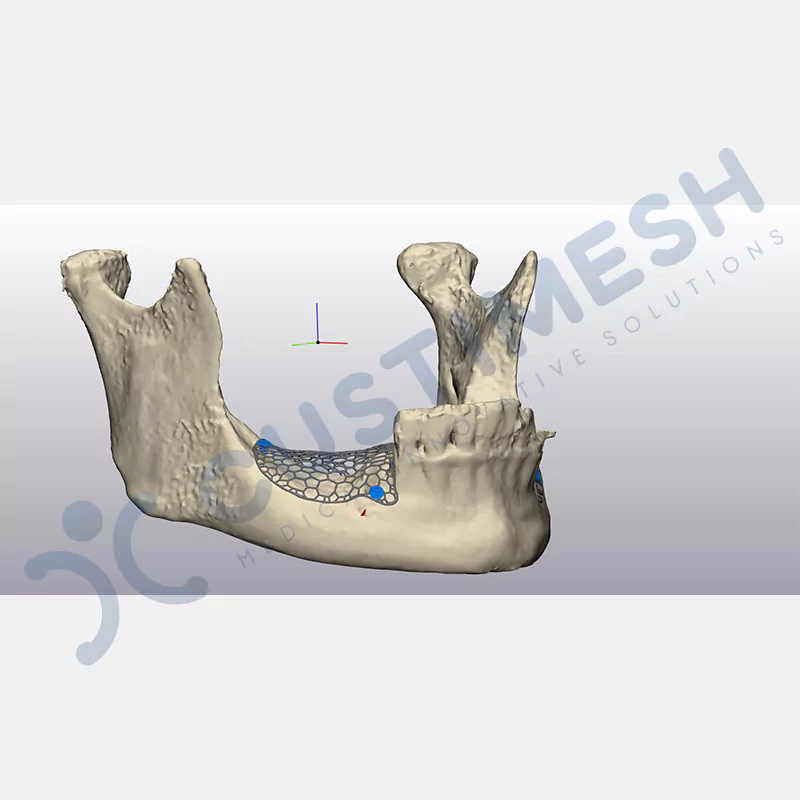
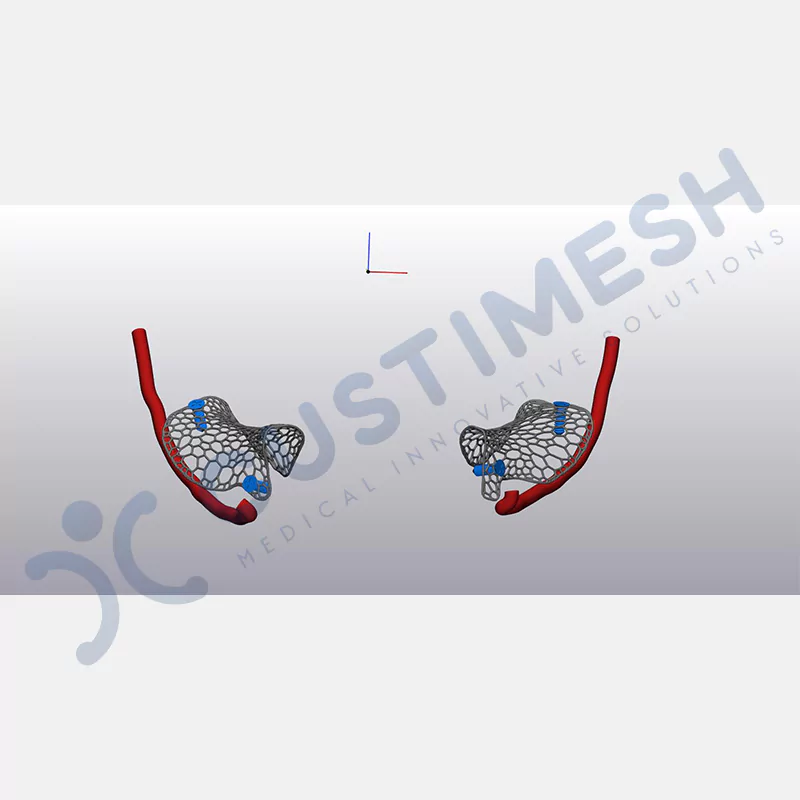
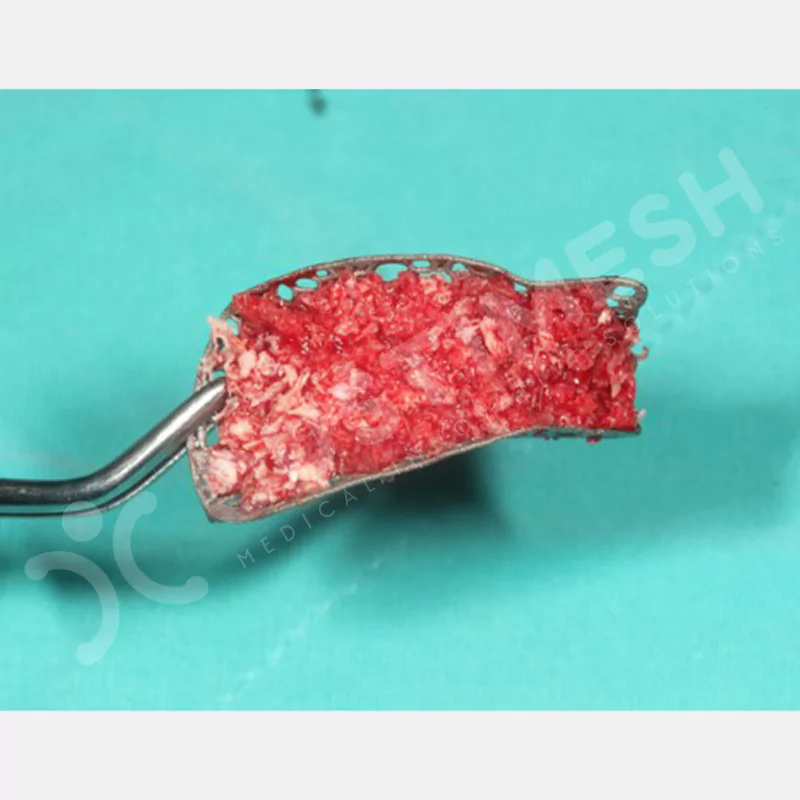
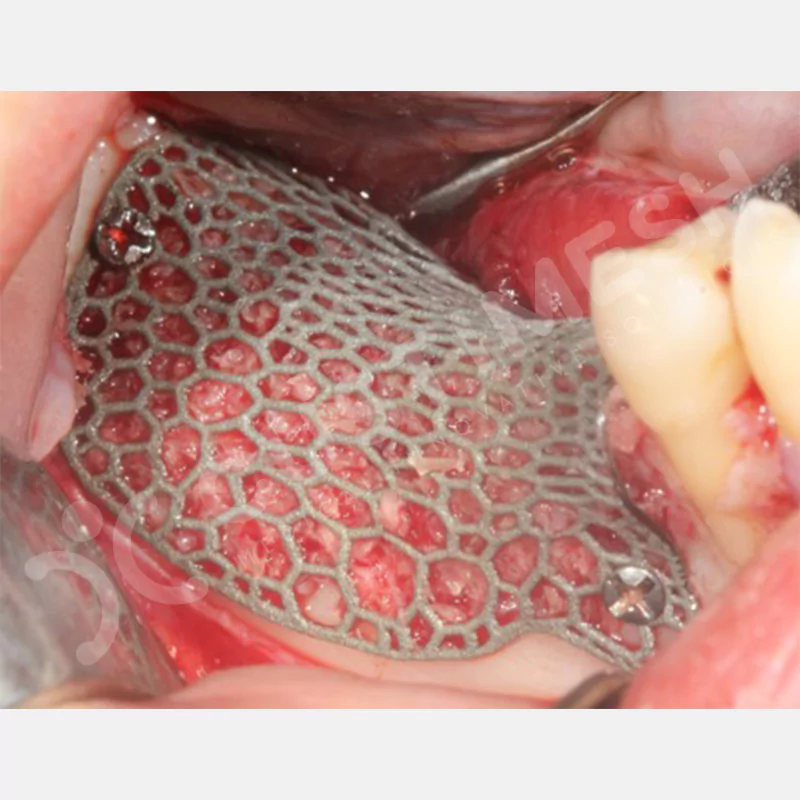
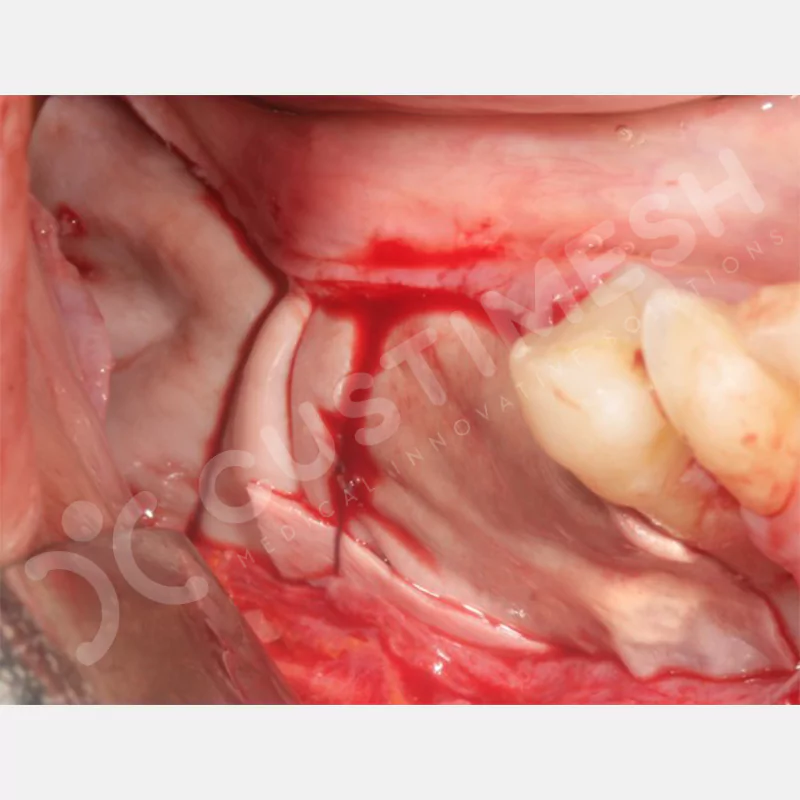
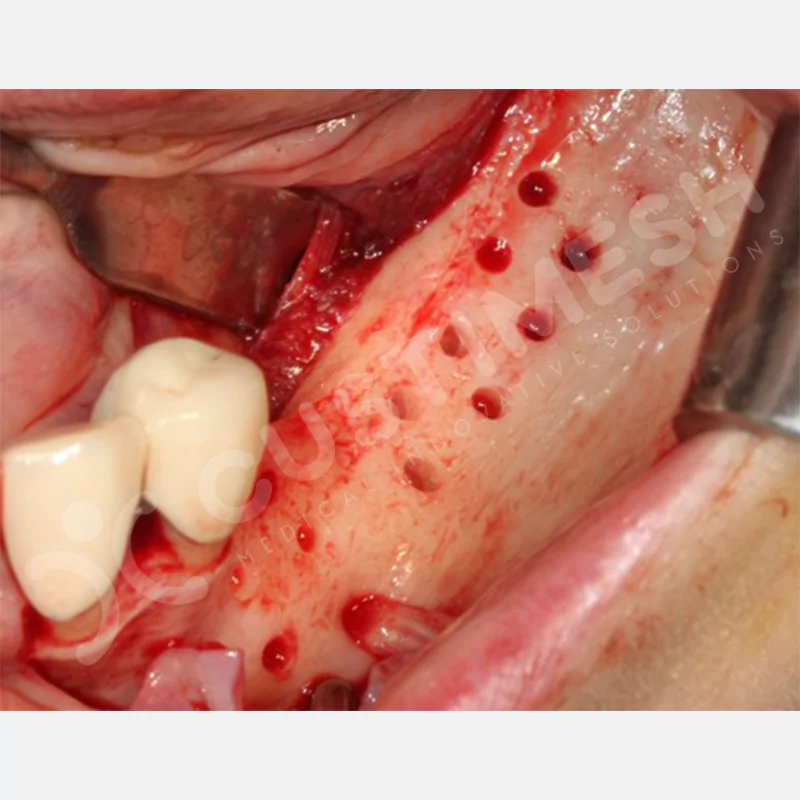
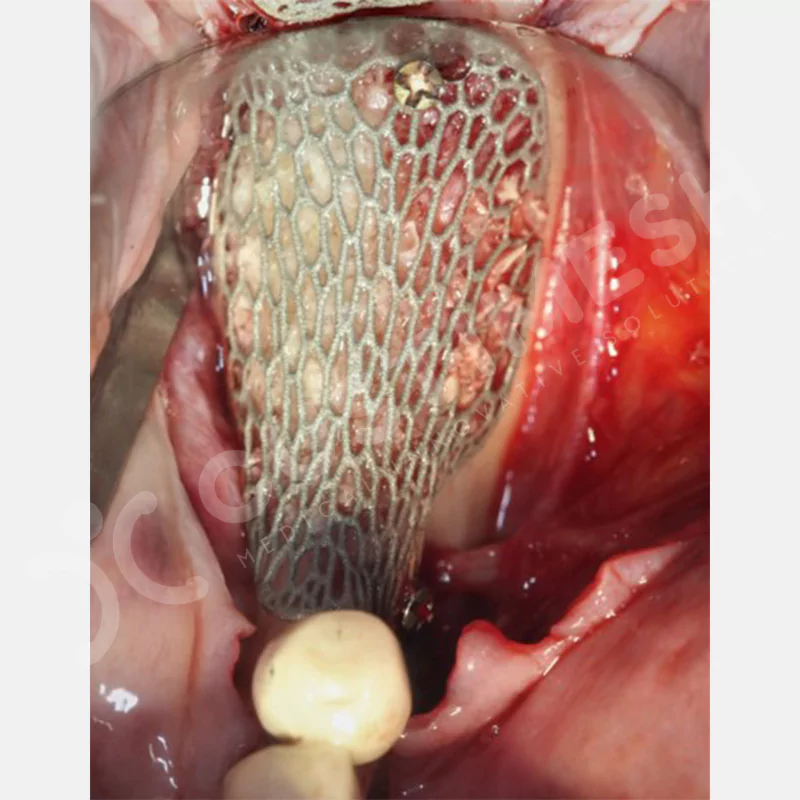
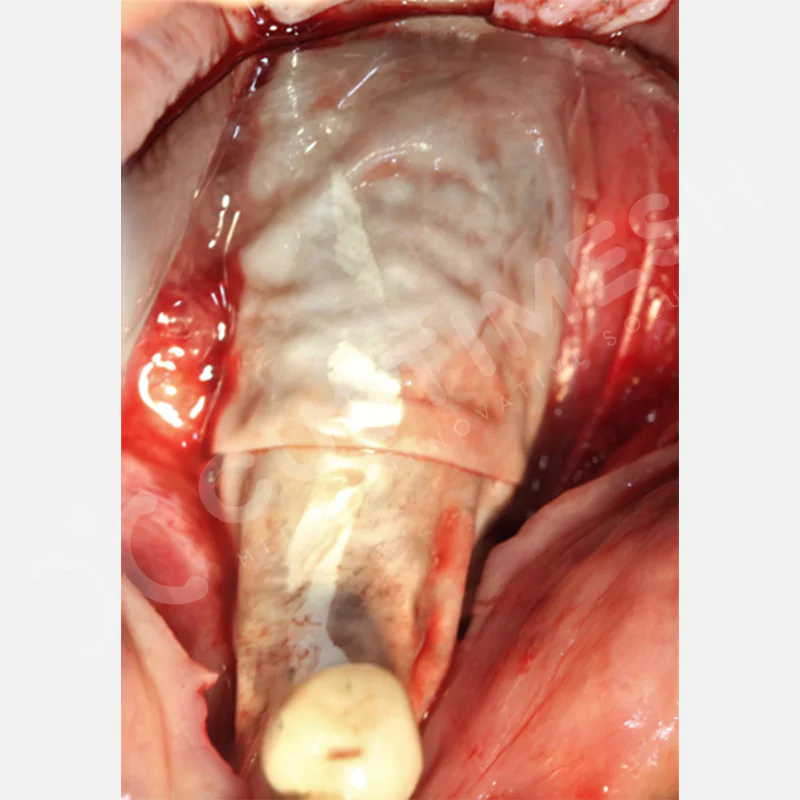
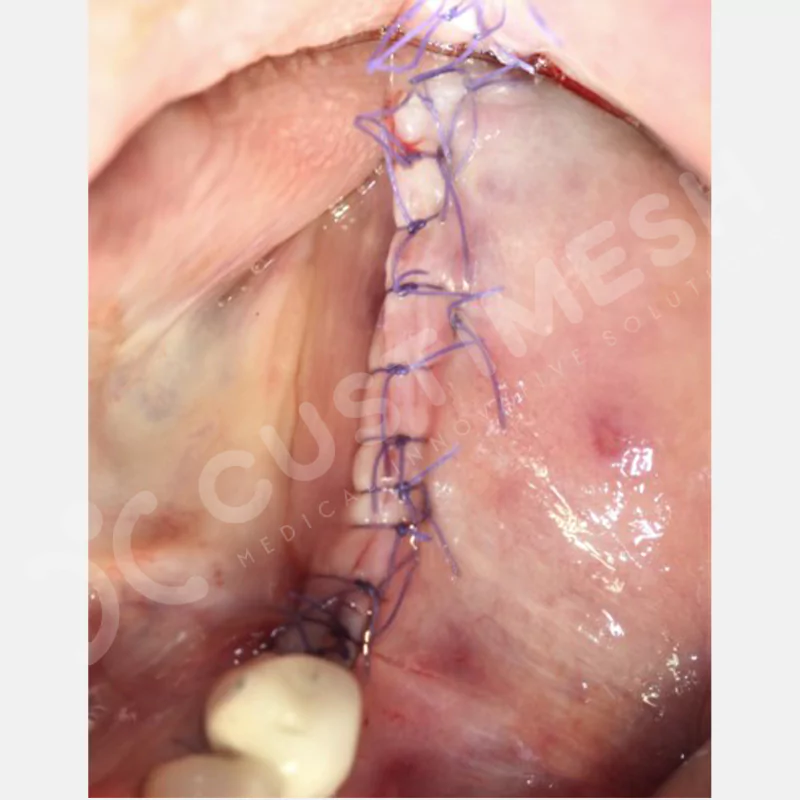
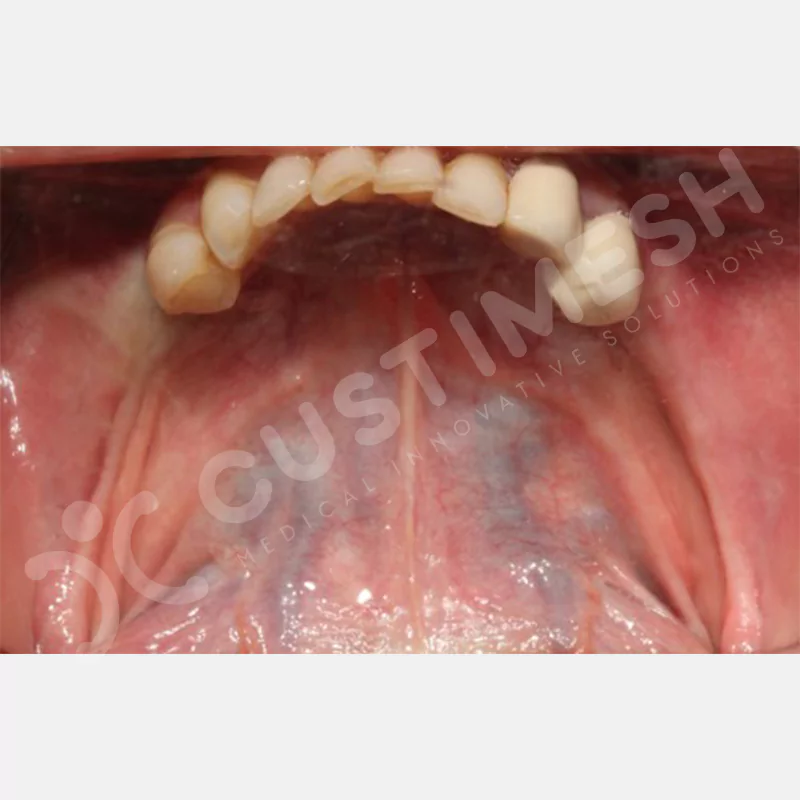
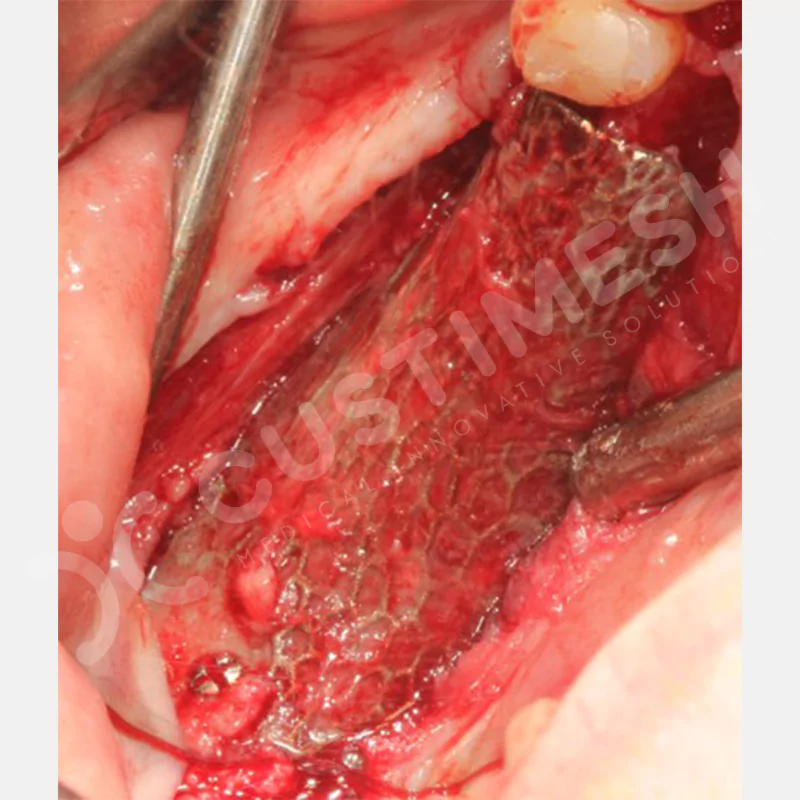
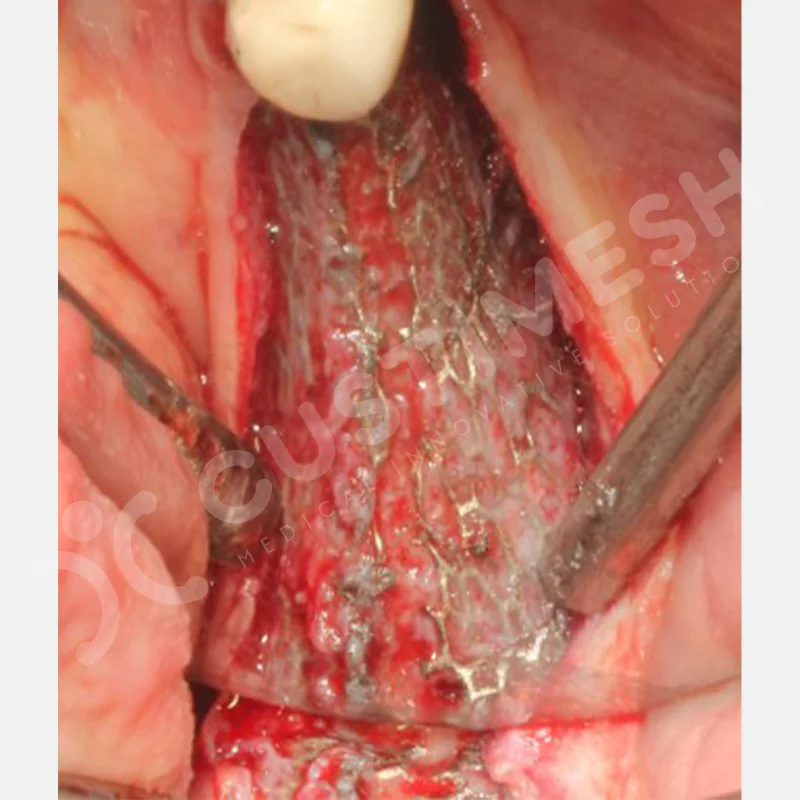
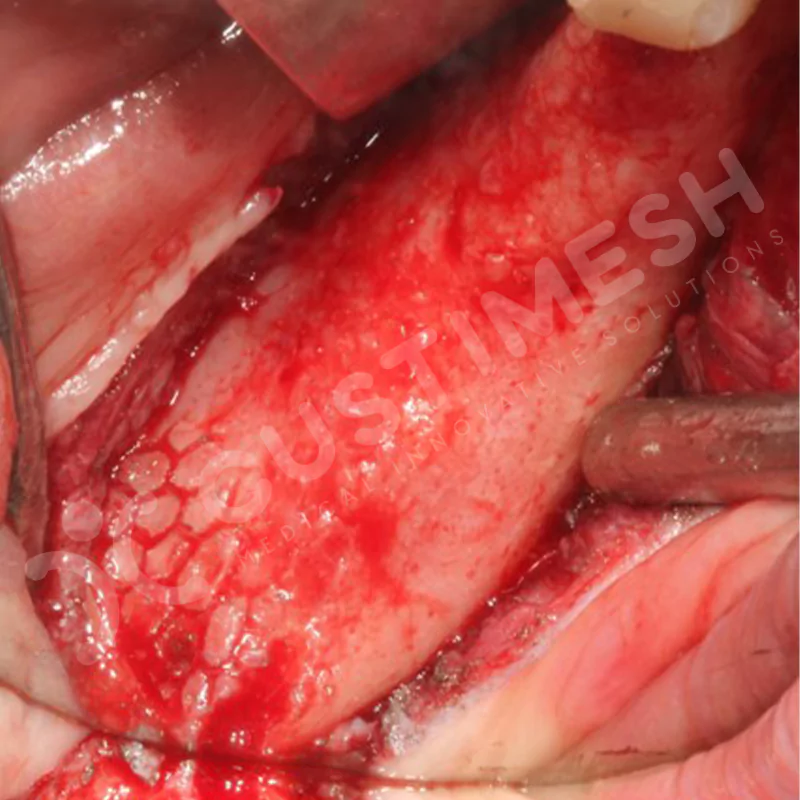
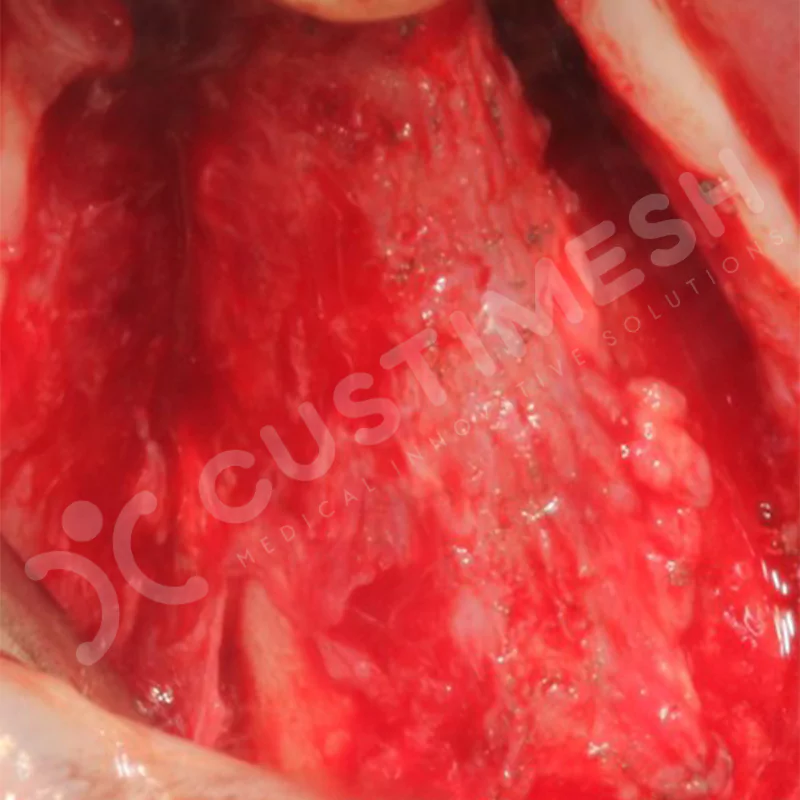
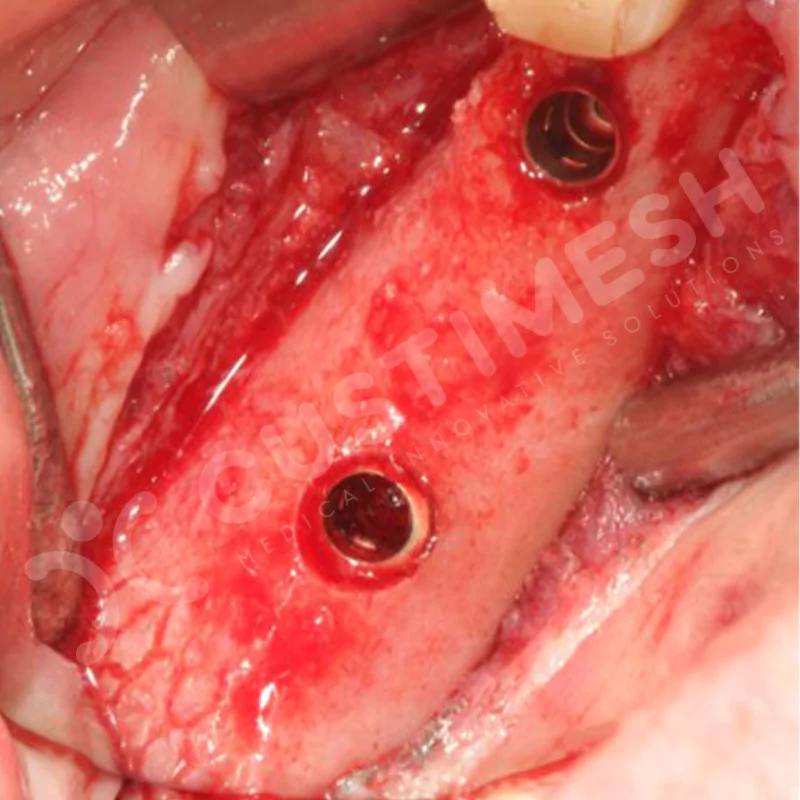
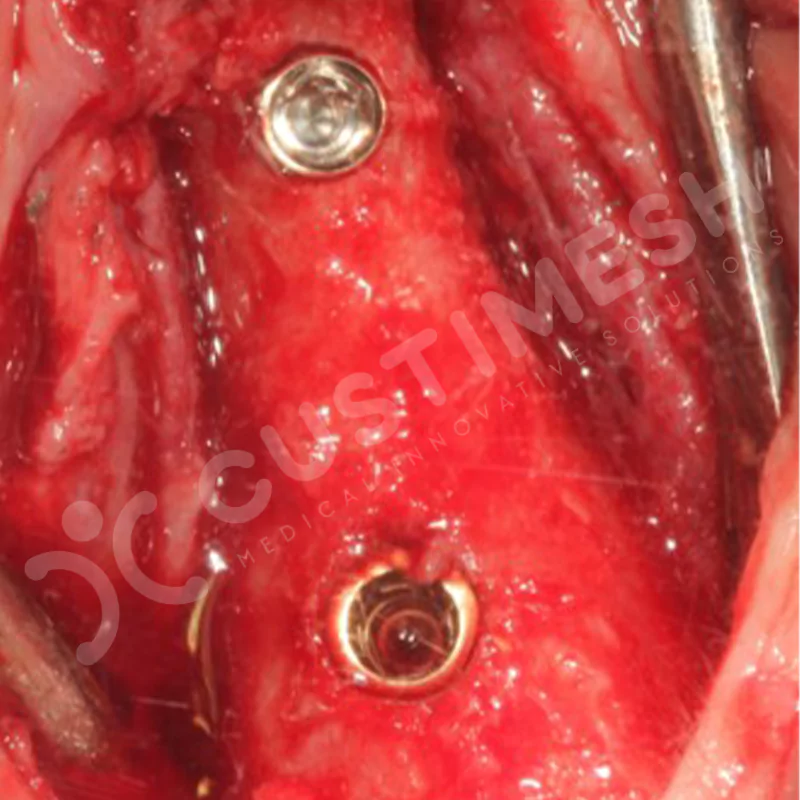
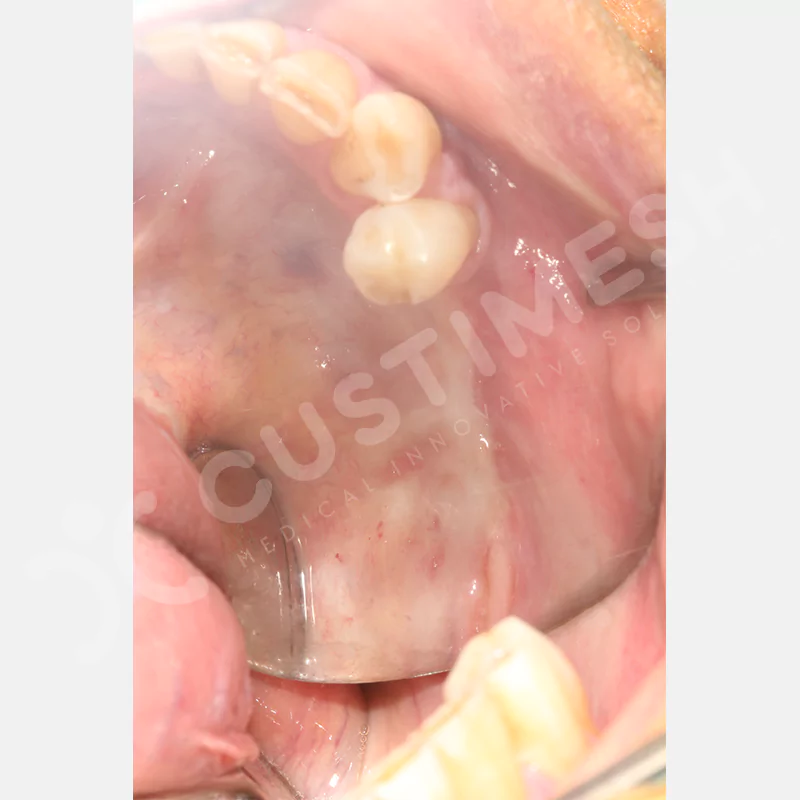
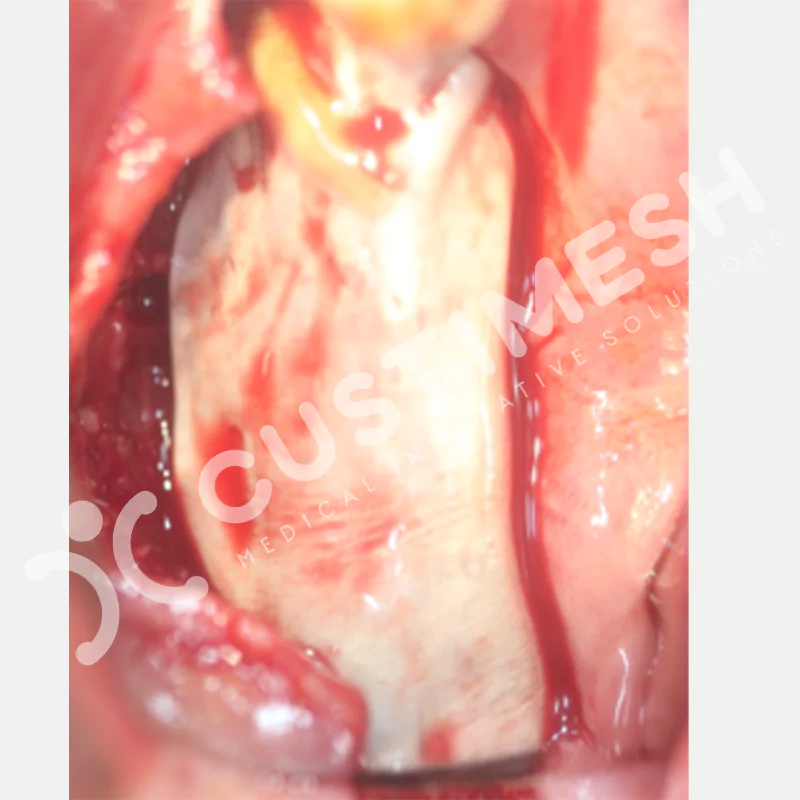
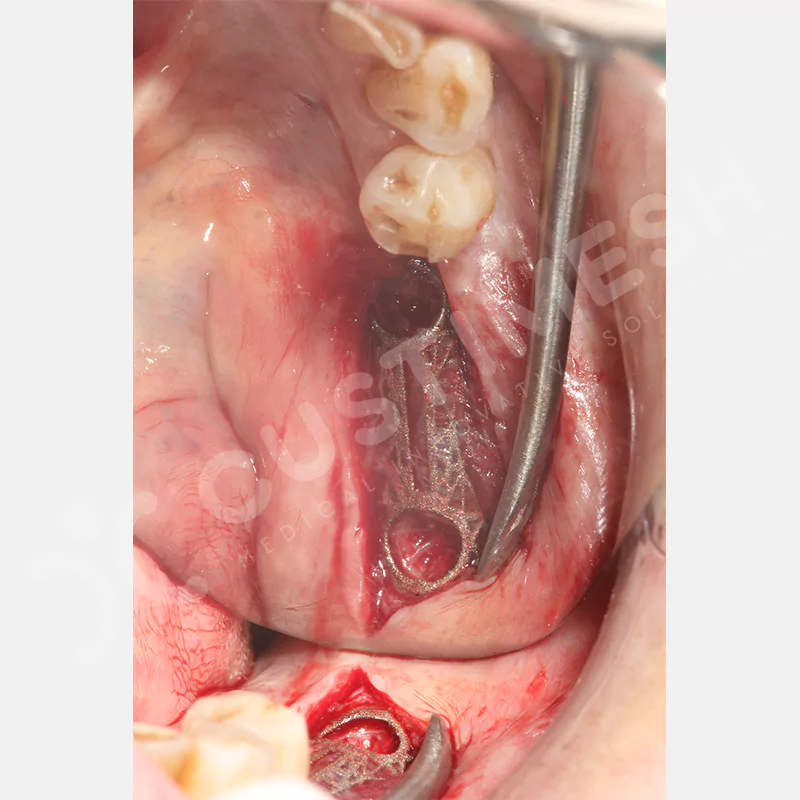
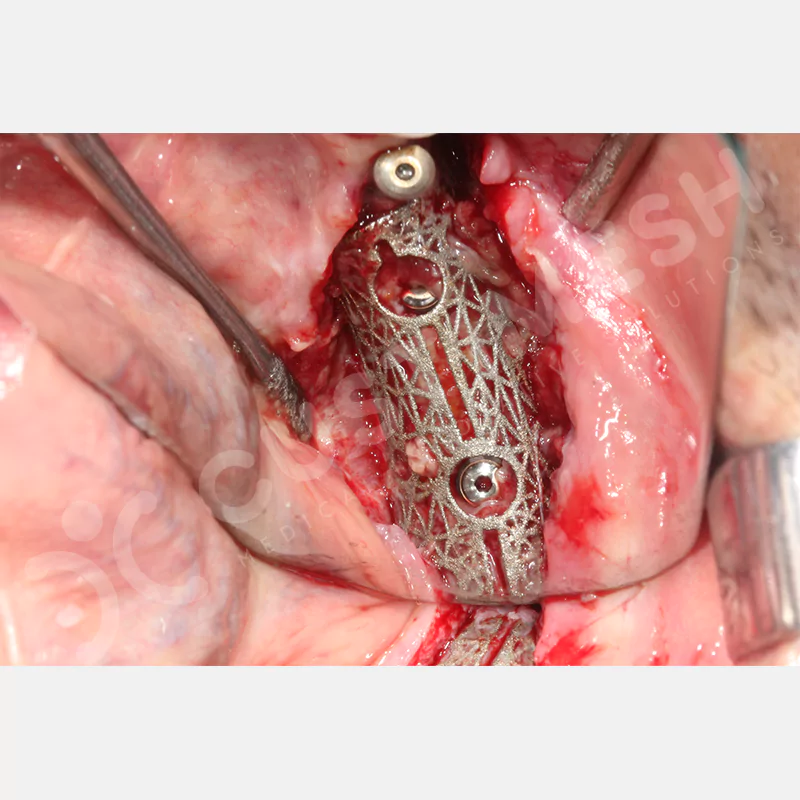
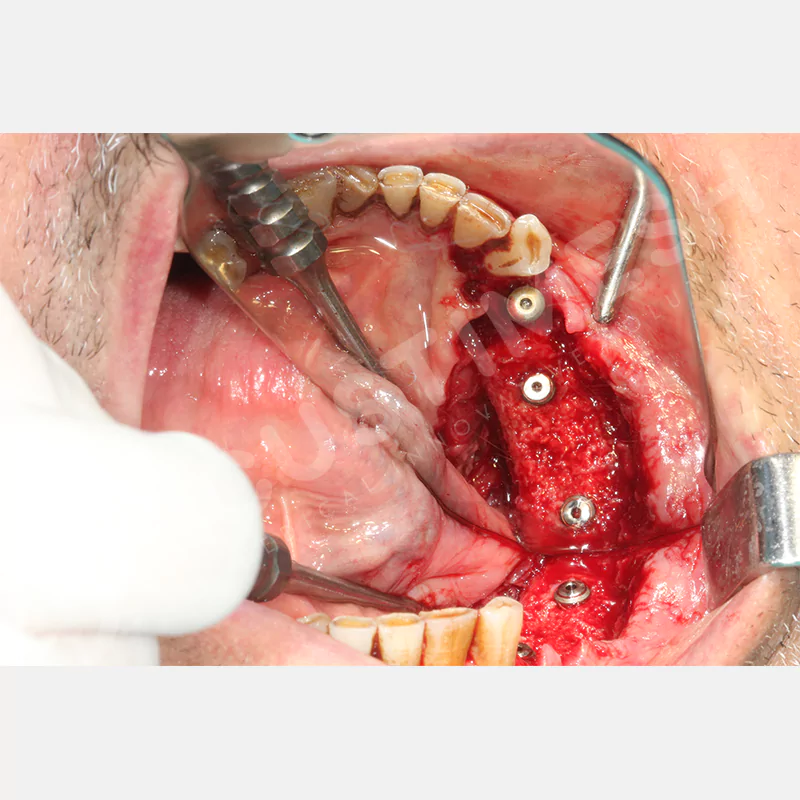
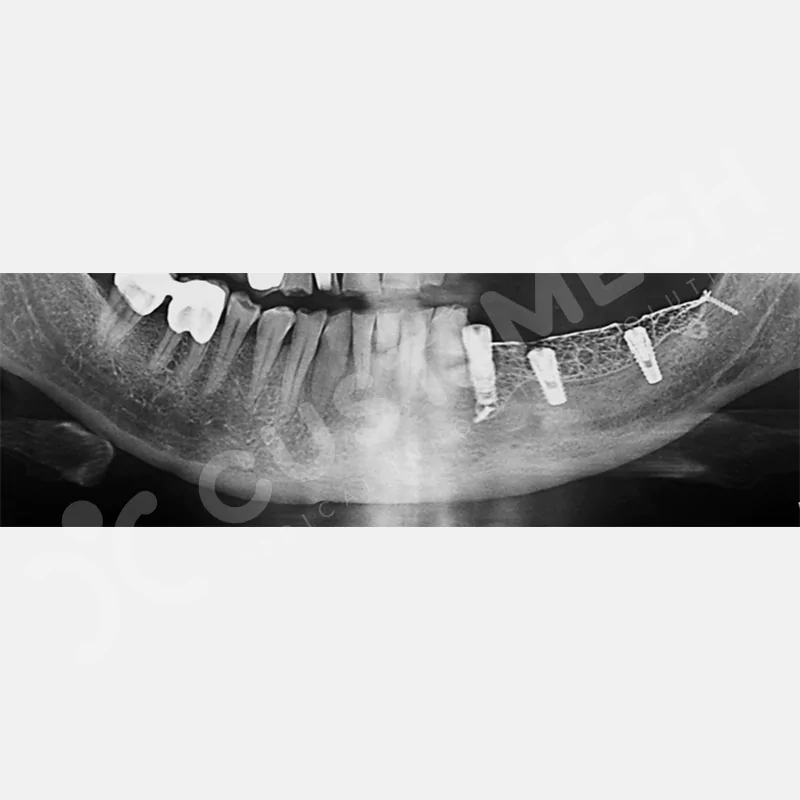
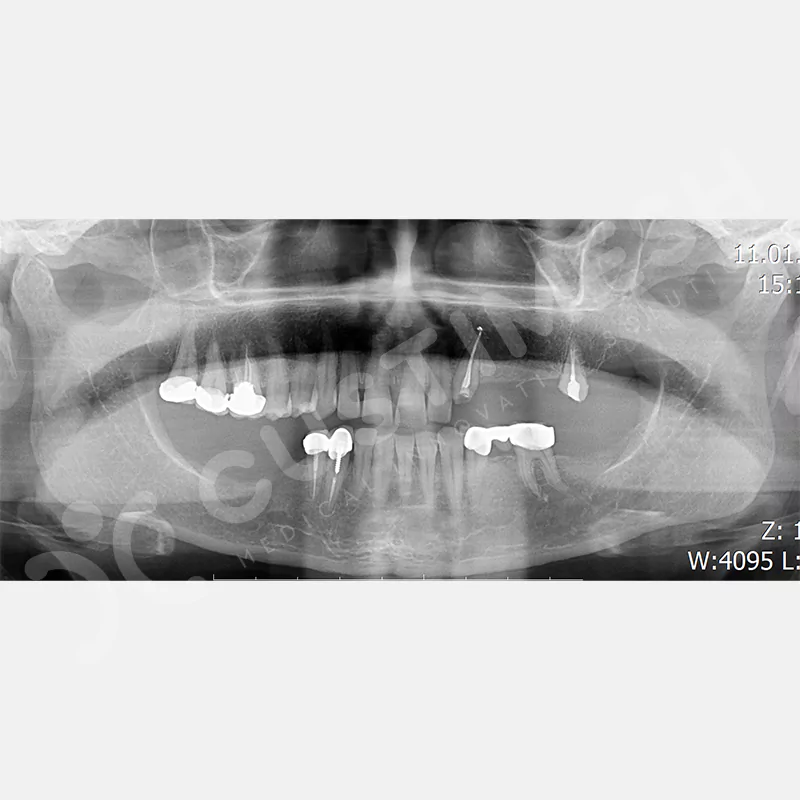
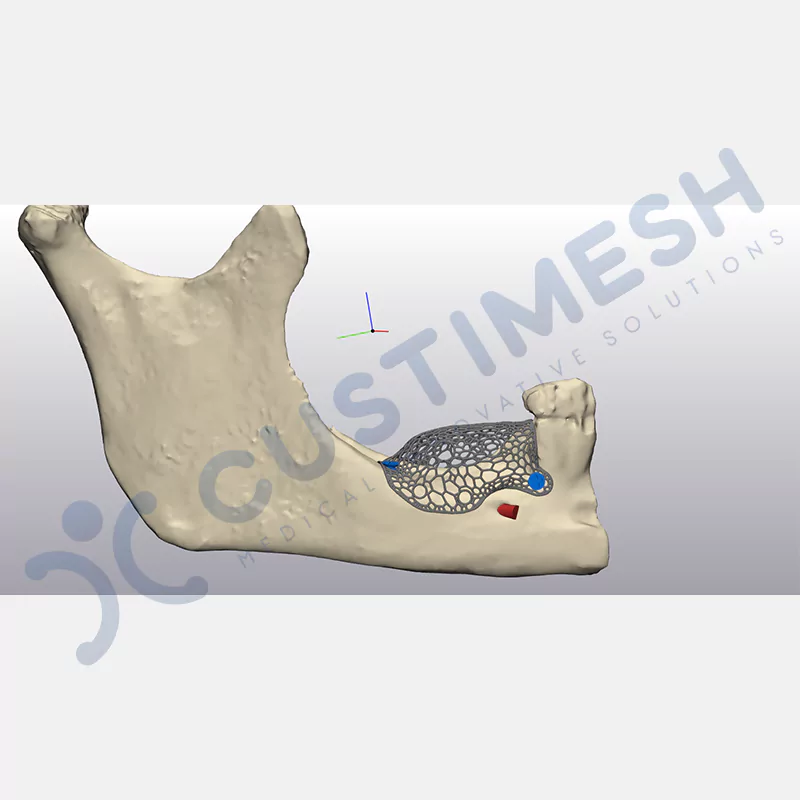
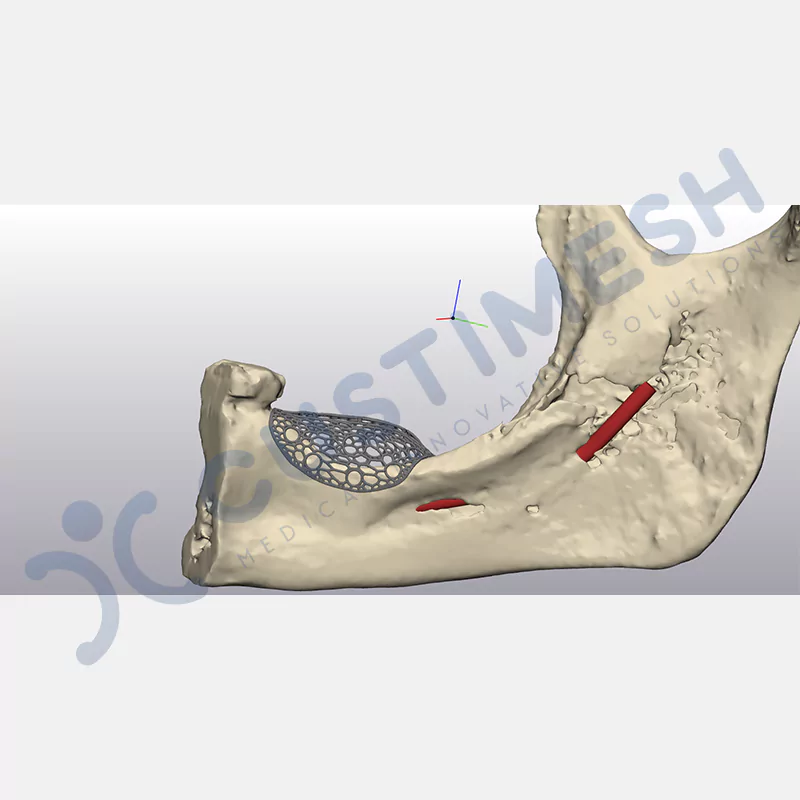
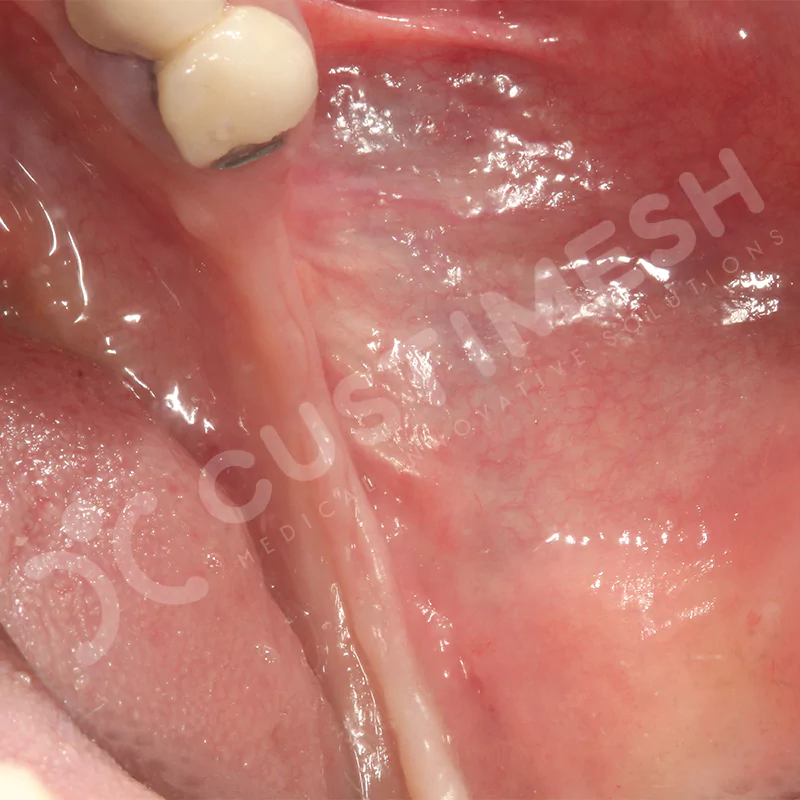
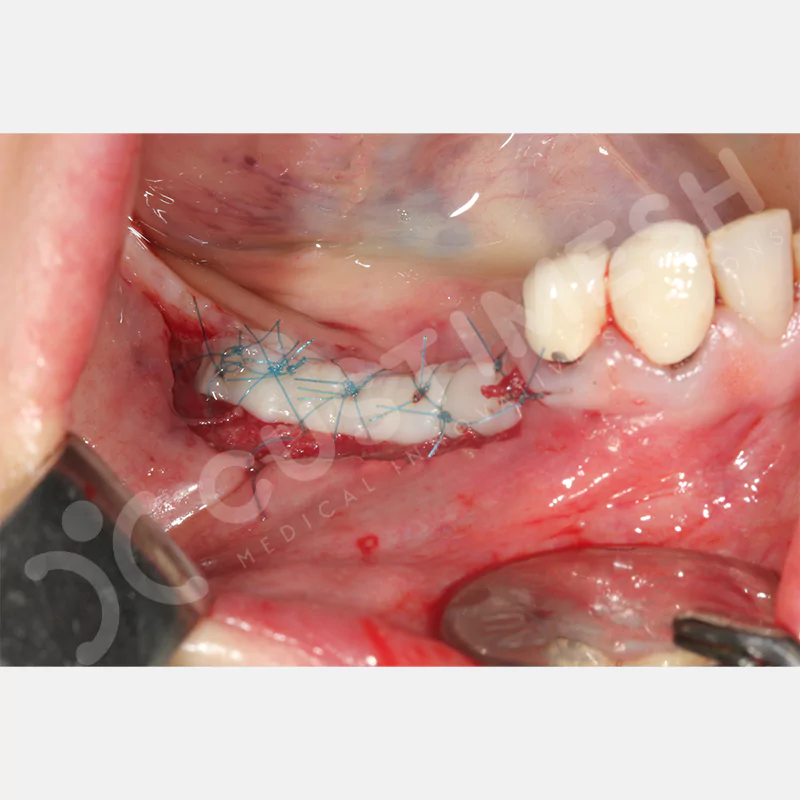
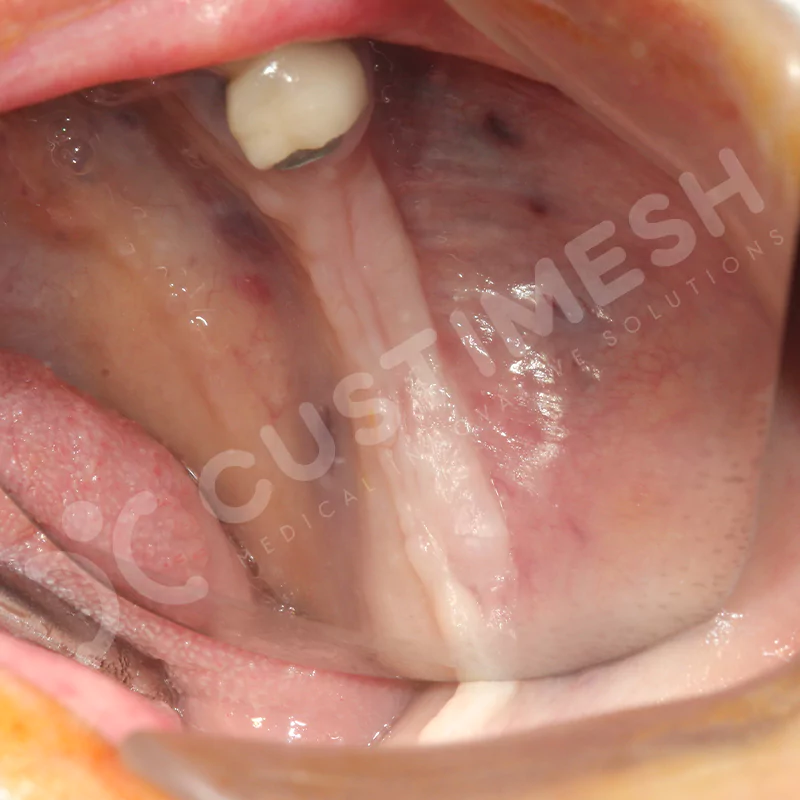
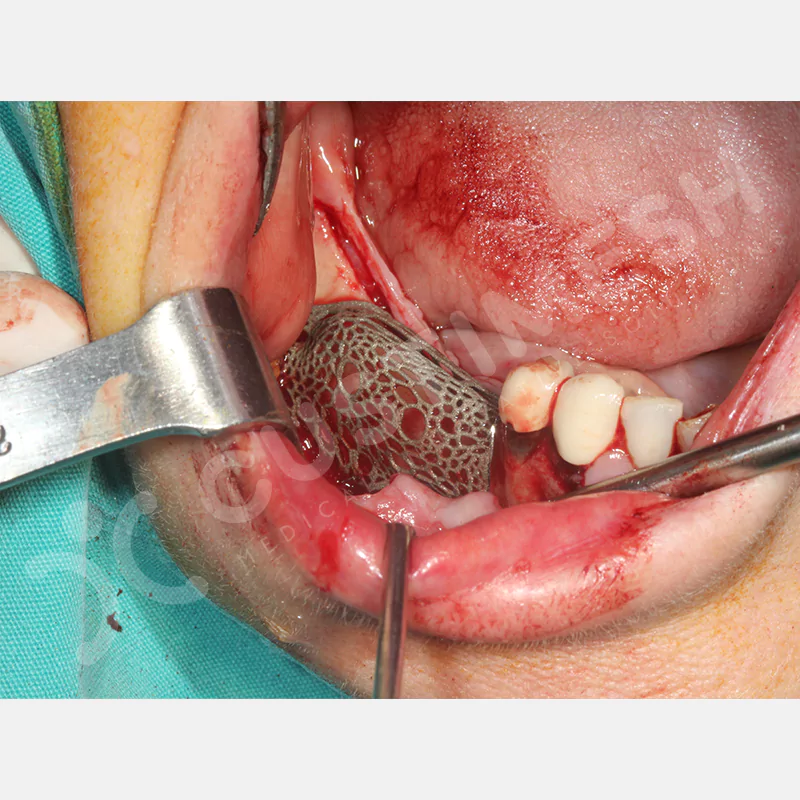
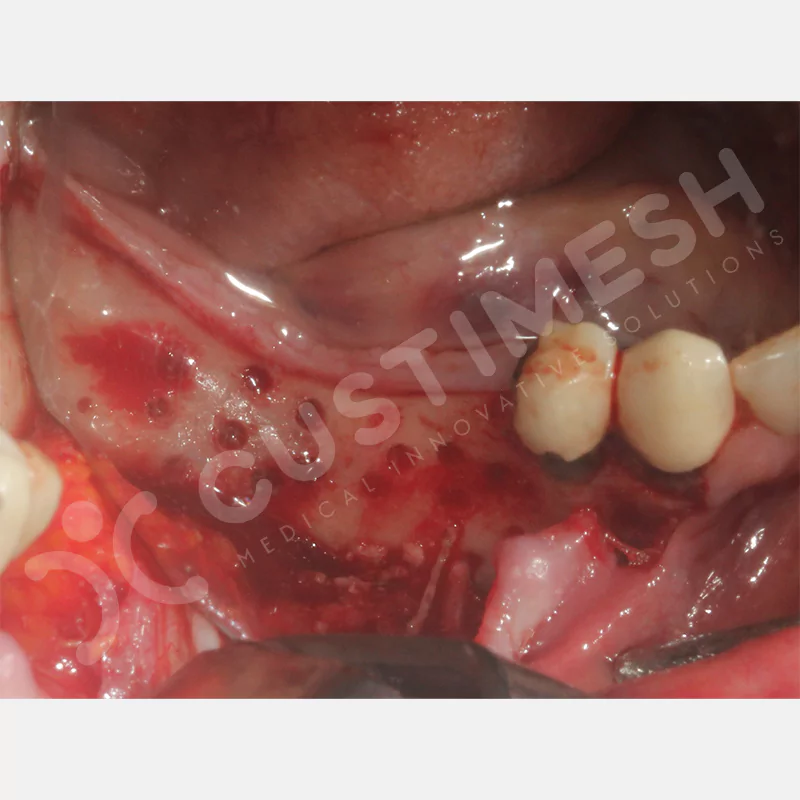
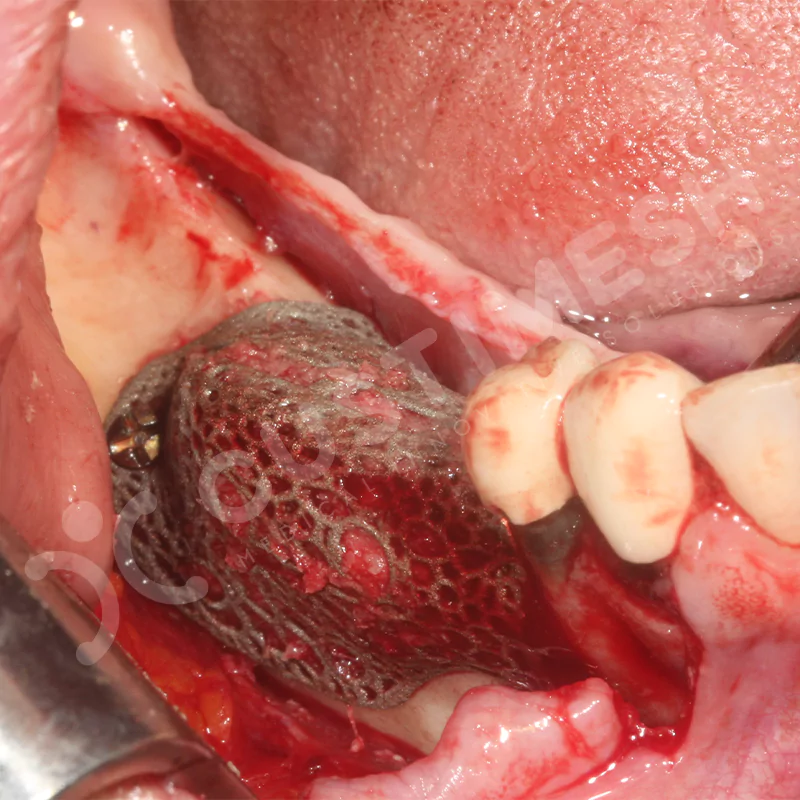
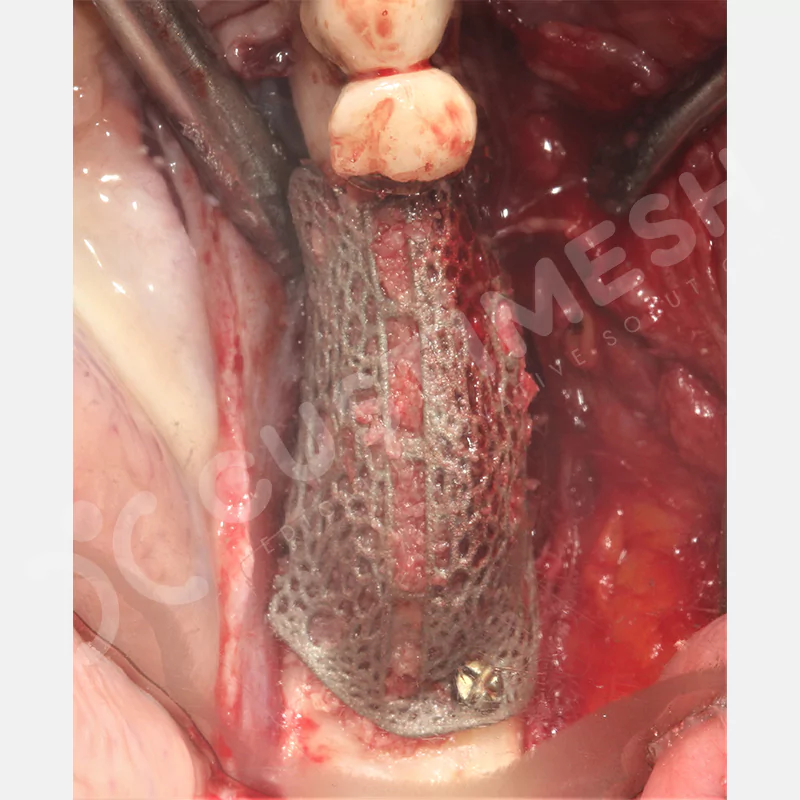
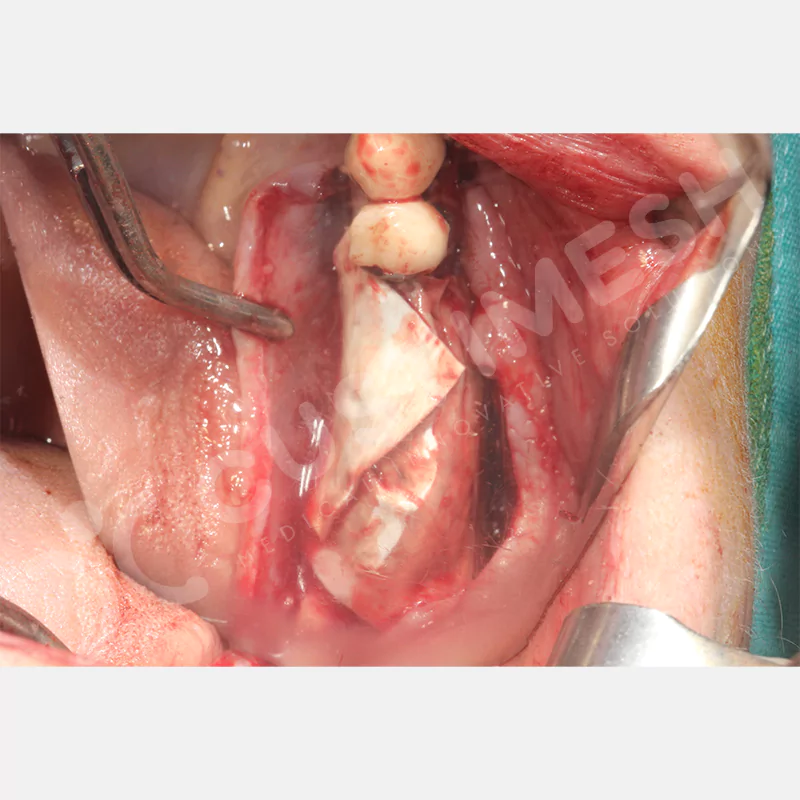
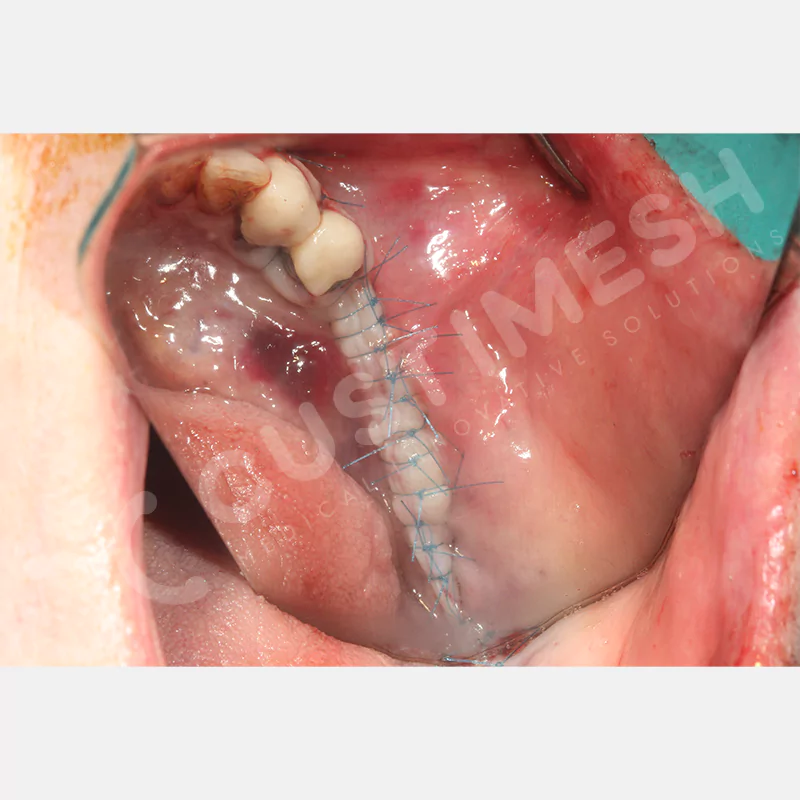
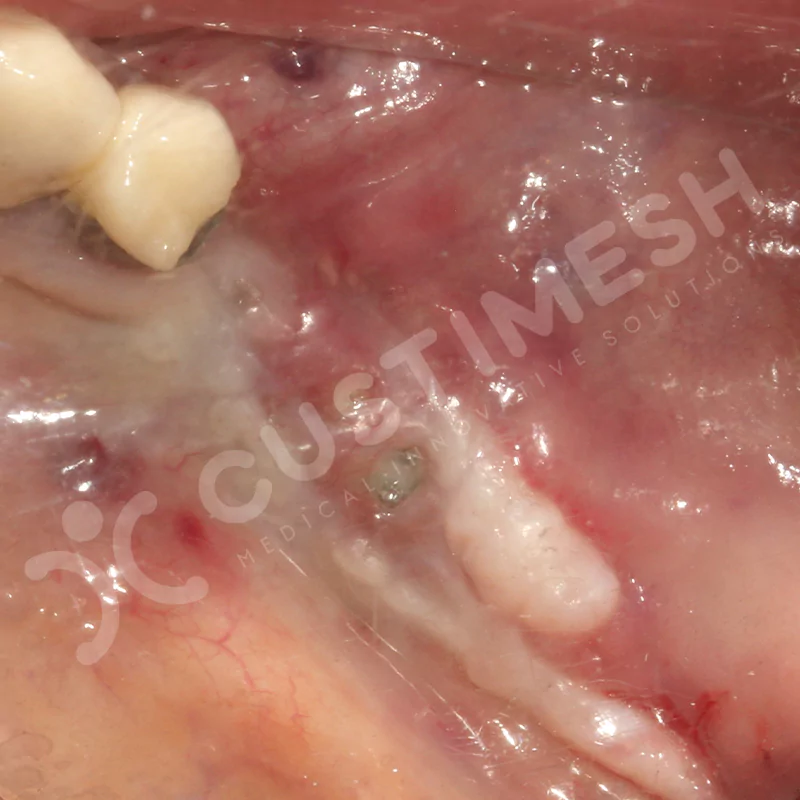
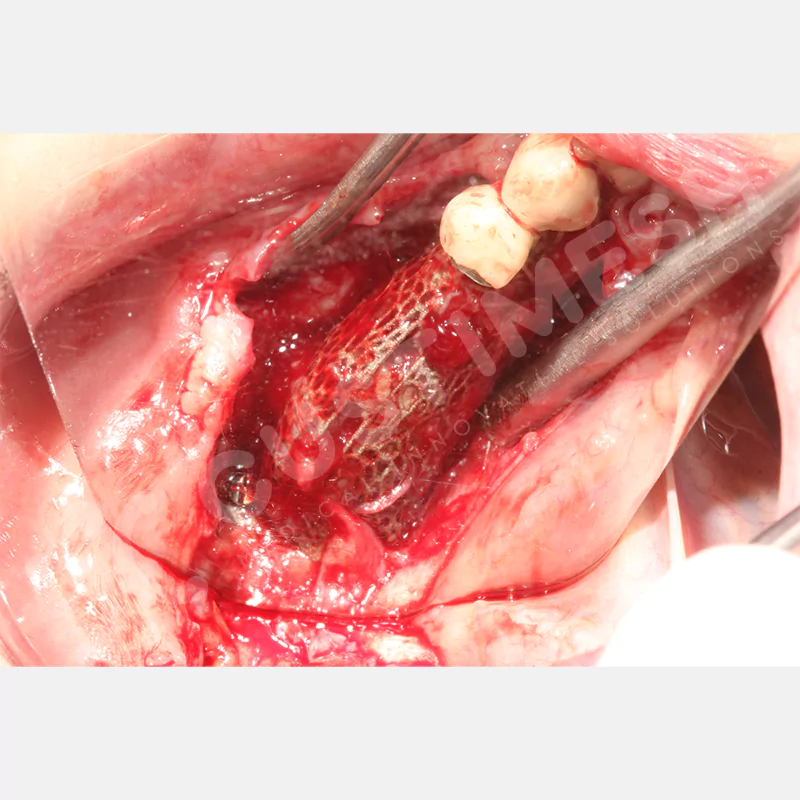
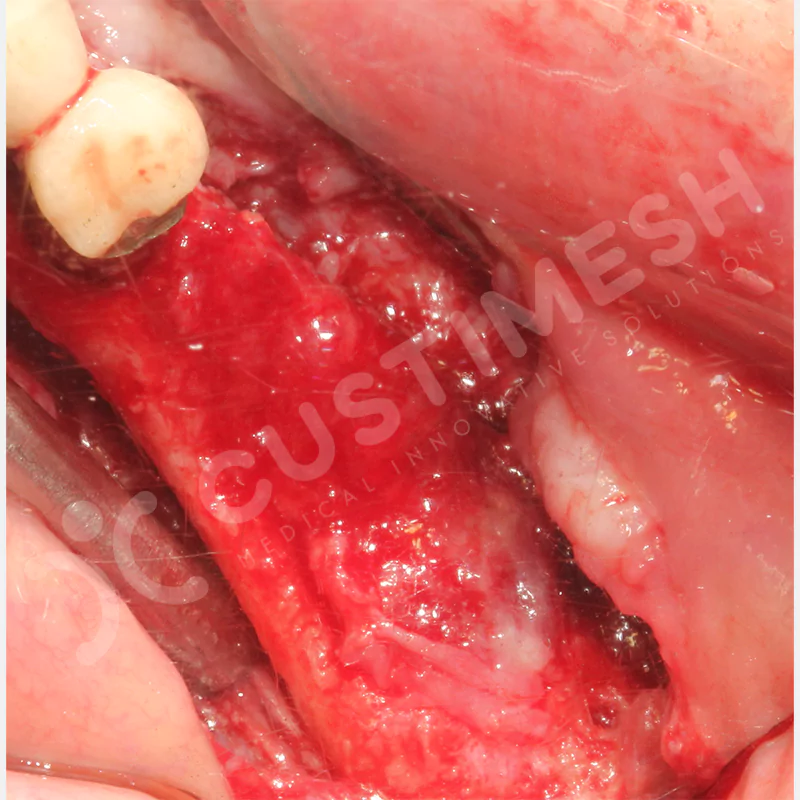
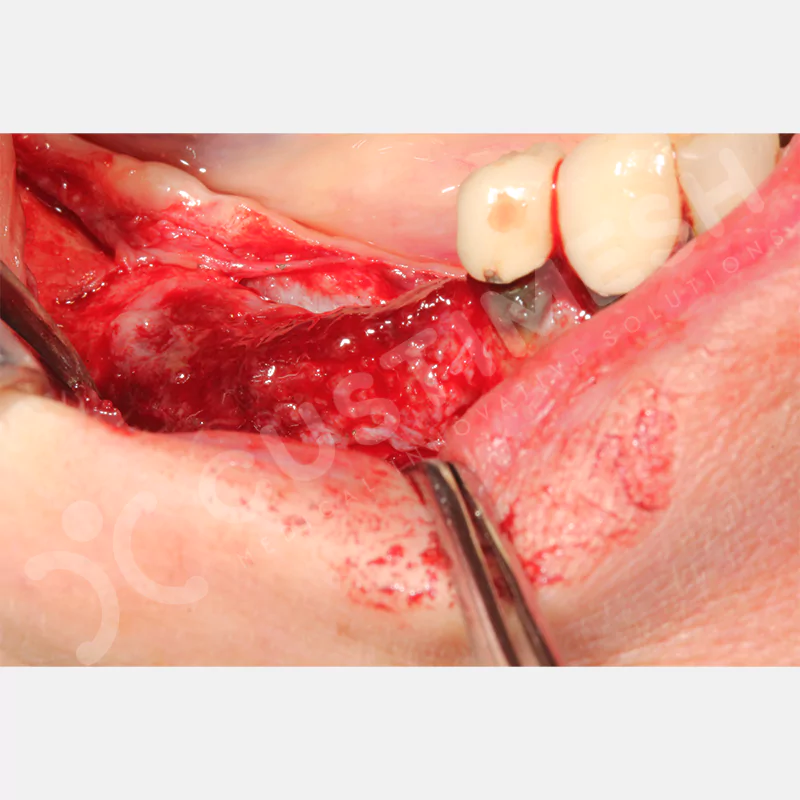
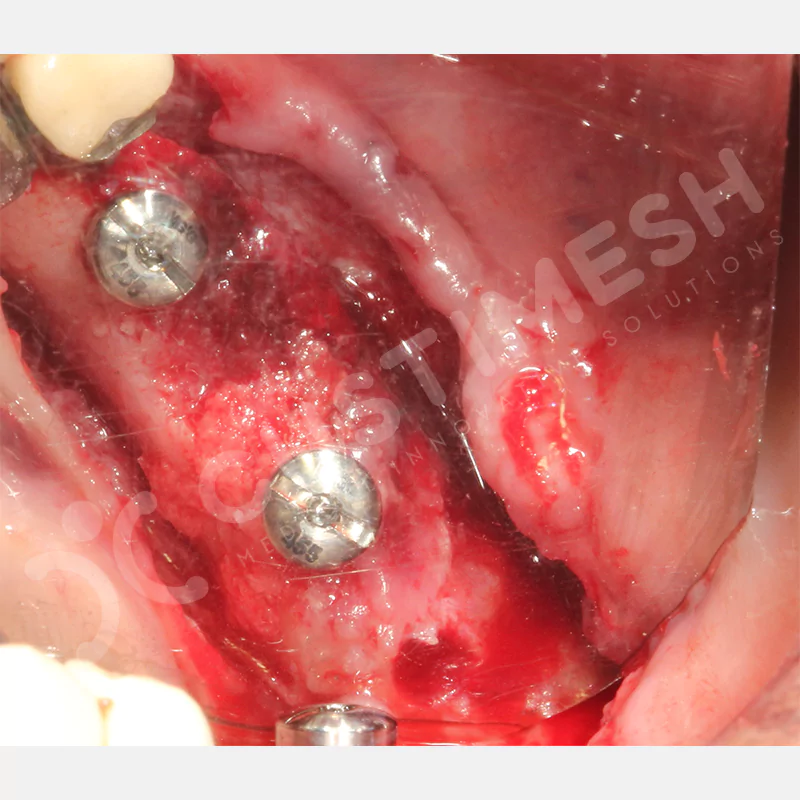
Developed to provide bone regeneration in patients who cannot receive dental implants, personalized titanium mesh is a titanium barrier system that is fully compatible with the patient’s own anatomy and is used to protect the graft material and support new bone formation in bone augmentation procedures.
Custom Titanium Mesh (CUSTIMESH)
Customized titanium mesh, developed to support bone regeneration in patients where dental implants cannot be applied, is a titanium barrier system perfectly adapted to the patient’s anatomy. It is used to preserve graft material and support new bone formation during bone augmentation procedures.
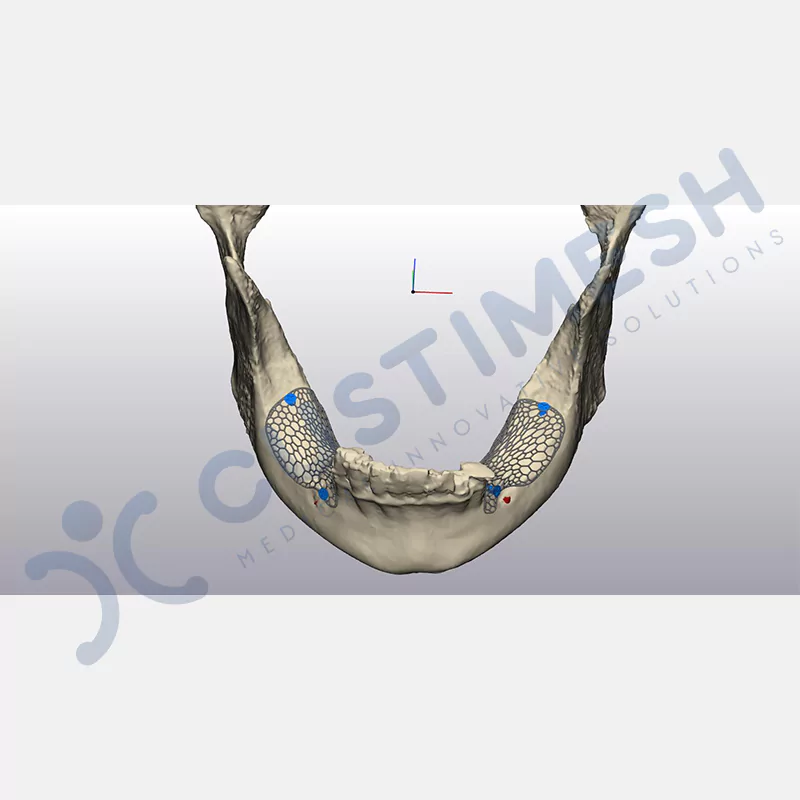
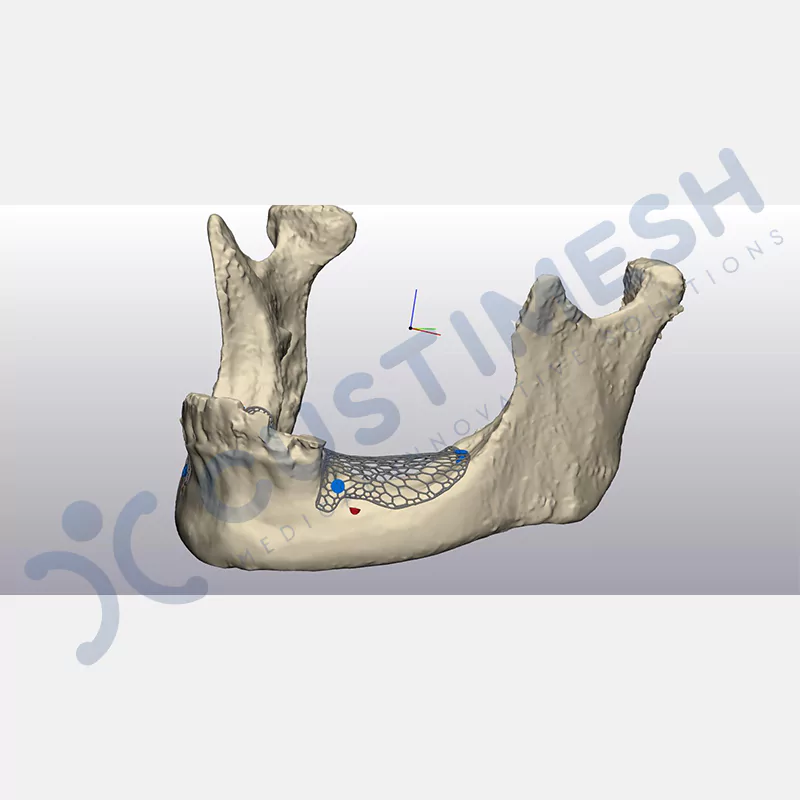
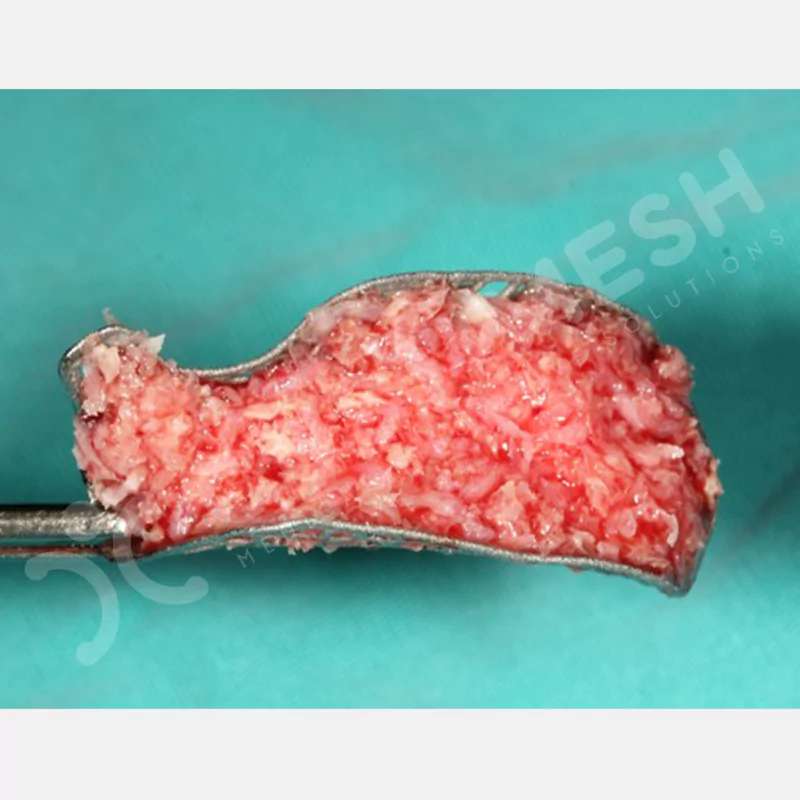
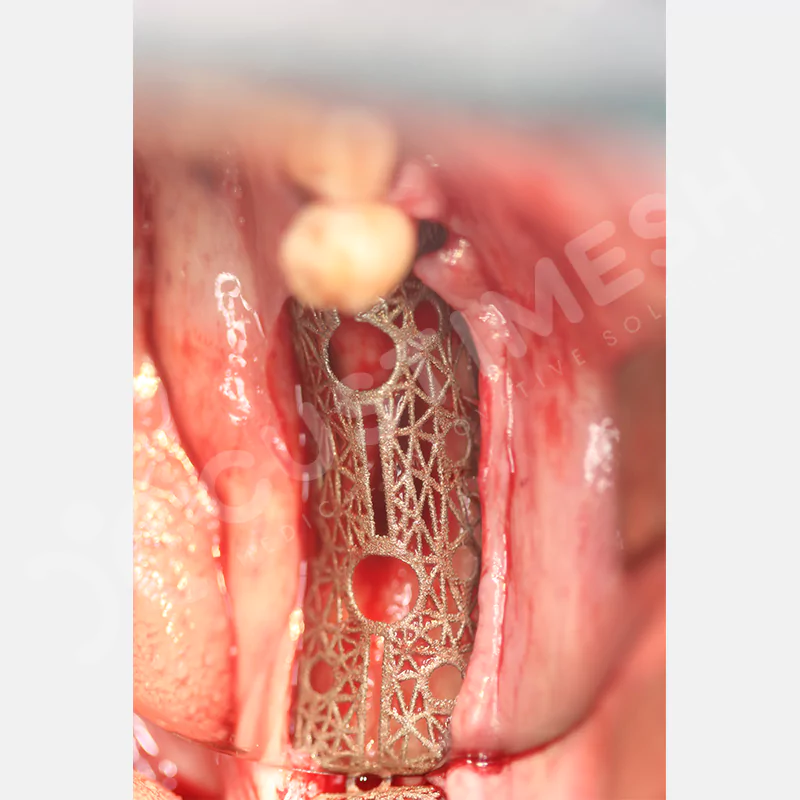
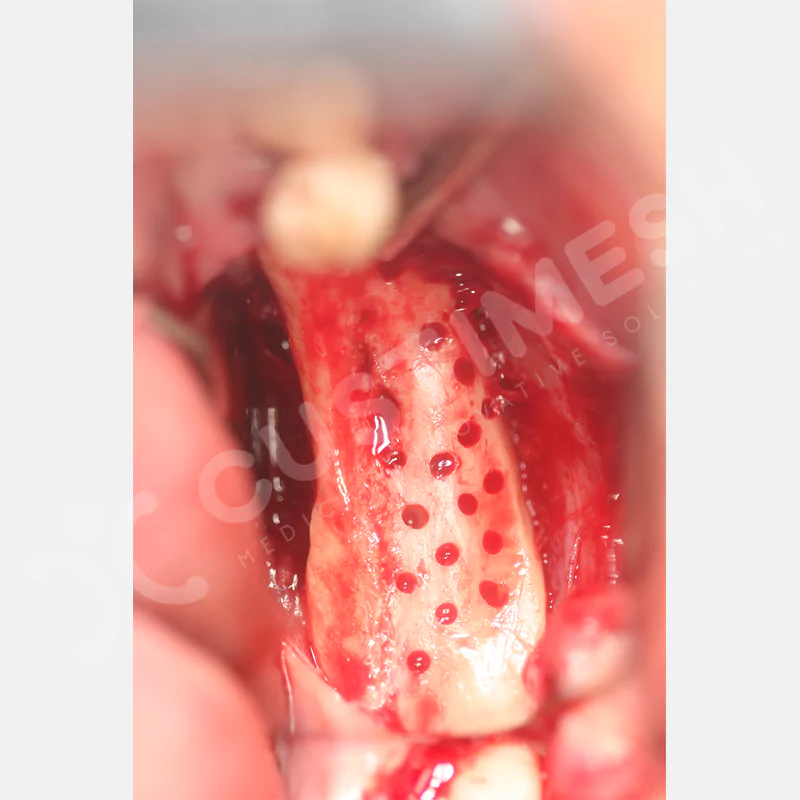
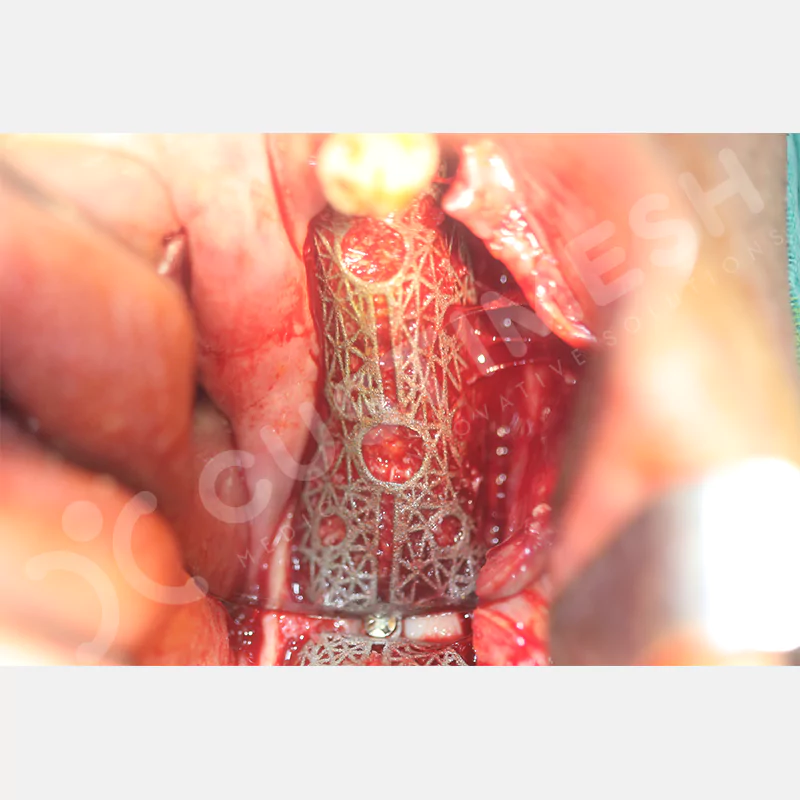
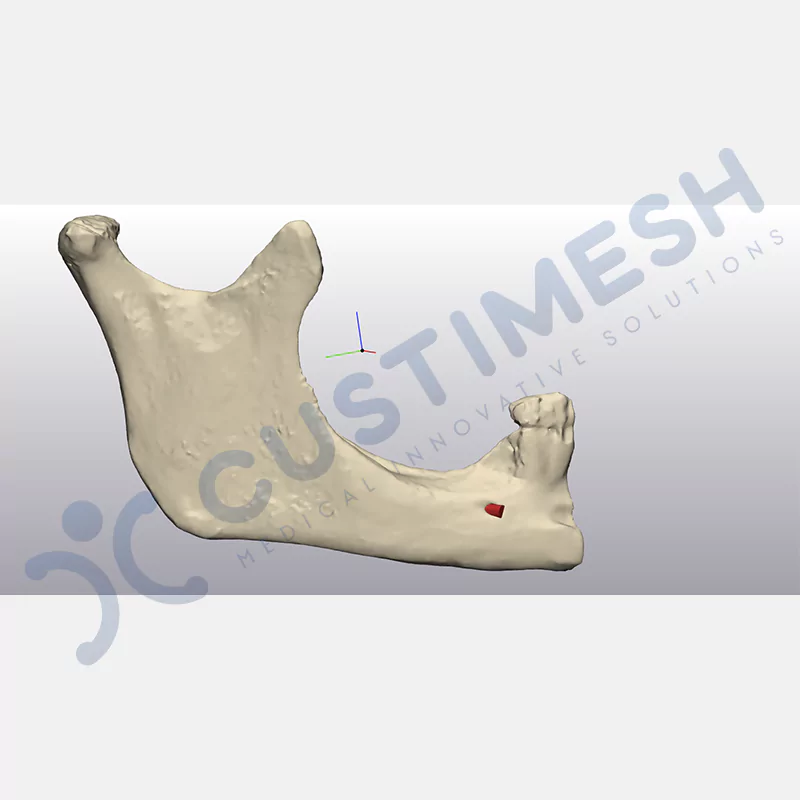
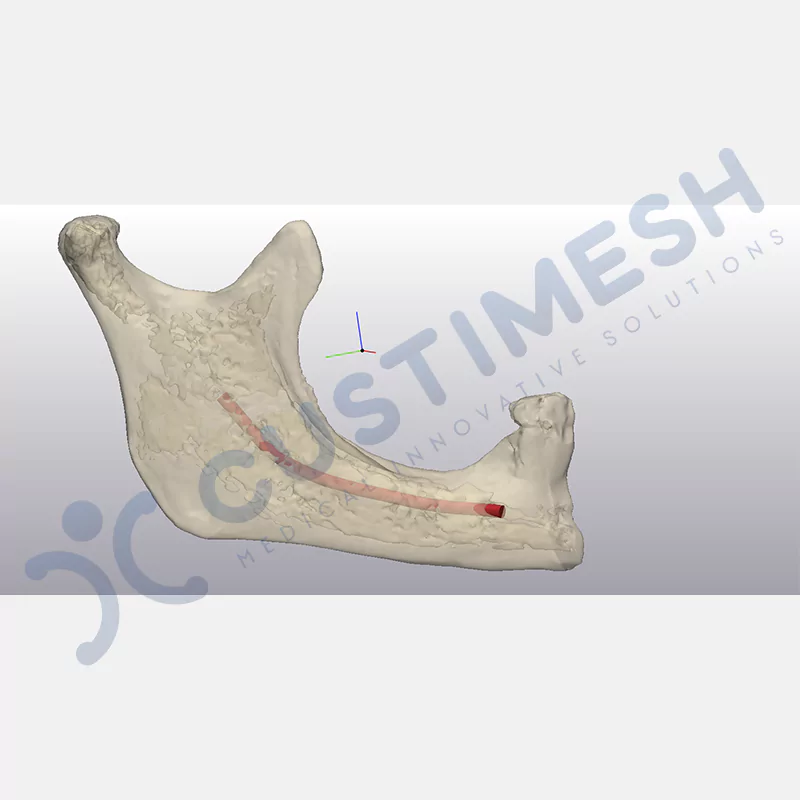
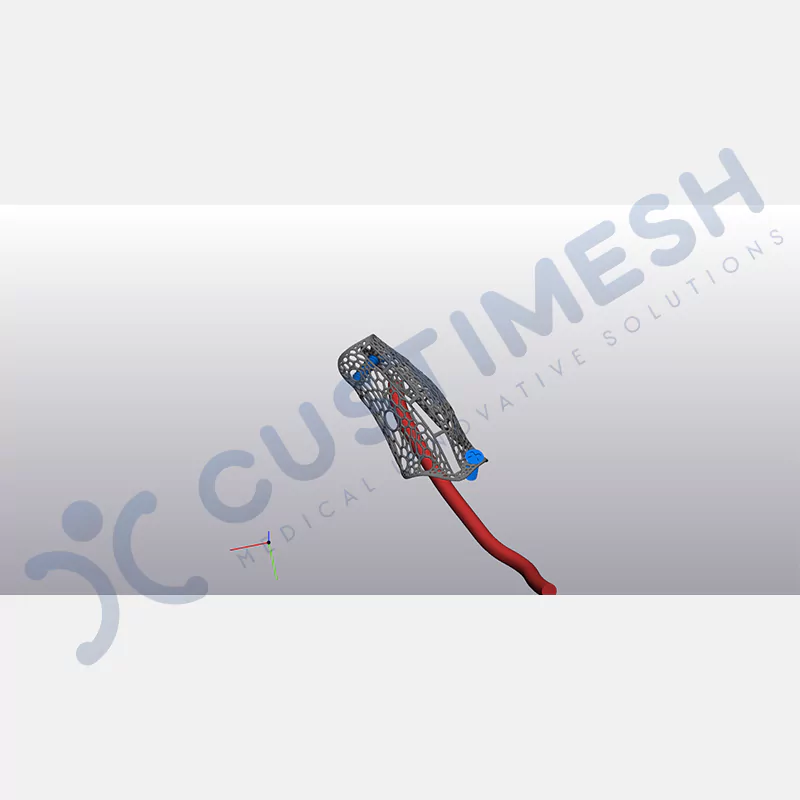
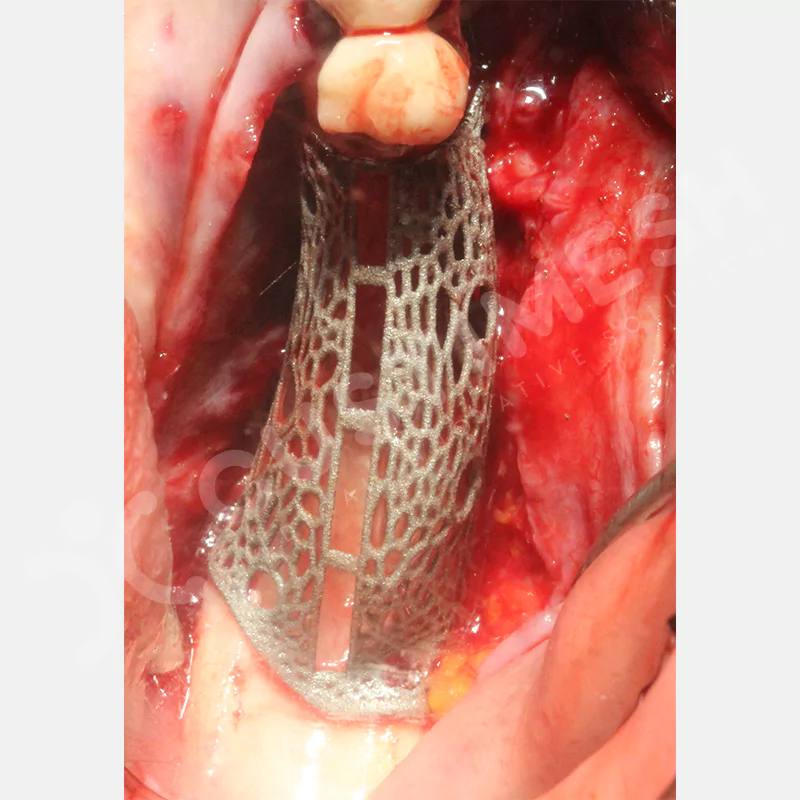
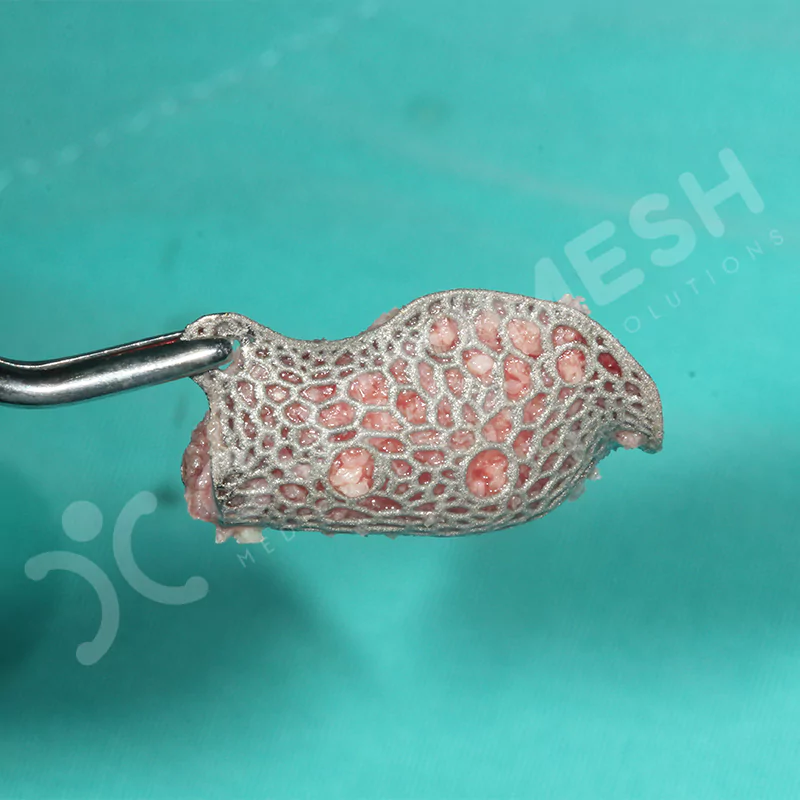
Made from medical-grade titanium, this innovative solution is designed using the patient’s CT data and CAD software, and produced with 3D printing technology. It offers superior mechanical stability and excellent adaptation properties, which standard meshes cannot provide, especially in complex bone defects. By perfectly fitting the anatomical structure, it ensures the graft material stays in place while supporting optimal vascularization to accelerate the bone regeneration process.
- Special Design
- Perfect Fit
- Grade 23 Medical Grade Titanium Material
- Optimally Designed Screw Holes
- Custom-Designed Implant and Condensation Holes
- Reduction in Surgical Time
- Custom Design for Easy Removal
- No Need for Screwing from the Palatal or Lingual Side
- Low Risk of Complications
CUSTIMESH | Order and Delivery Process
- DICOM The patient’s DICOM file must be sent via WeTransfer to custimesh.case@gmail.com. The physician’s name and the specific region for the Custimplant must be clearly stated in the description section. Prior to creating the Custimplant, a total prosthesis containing barium sulfate must be fabricated for the patient. Then, a Medical CT scan should be performed while the patient is in the occlusion position, using a slice thickness of 0.5 mm.
- DOCTOR A 3D model is prepared based on the region where the Custimplant is to be planned. The model is then submitted for the physician’s approval, and communication is established with the physician for any necessary evaluations.
- PAYMENT At this stage, payment is made, and the active design process begins, initiating the Custimplant design phase.
- DESIGN Preliminary evaluations and analyses are conducted for the relevant region. The design of the Custimplant is then finalized by determining the positions of the multi-units, prosthetic emergence profiles, safe screw locations, and optimal intraoral screw access angles.
- DOCTOR APPROVAL The final productionready design of the custom-made Custimplant is submitted for the physician’s approval.
- PRODUCTION The Custimplant approved by the physician is manufactured using 3D printing technology from Grade 23 (Ti6Al4V) Medical Titanium Alloy.
- SHIPPING After undergoing the necessary postprocessing stages, the Custimplant is carefully prepared and shipped to the respective physician.
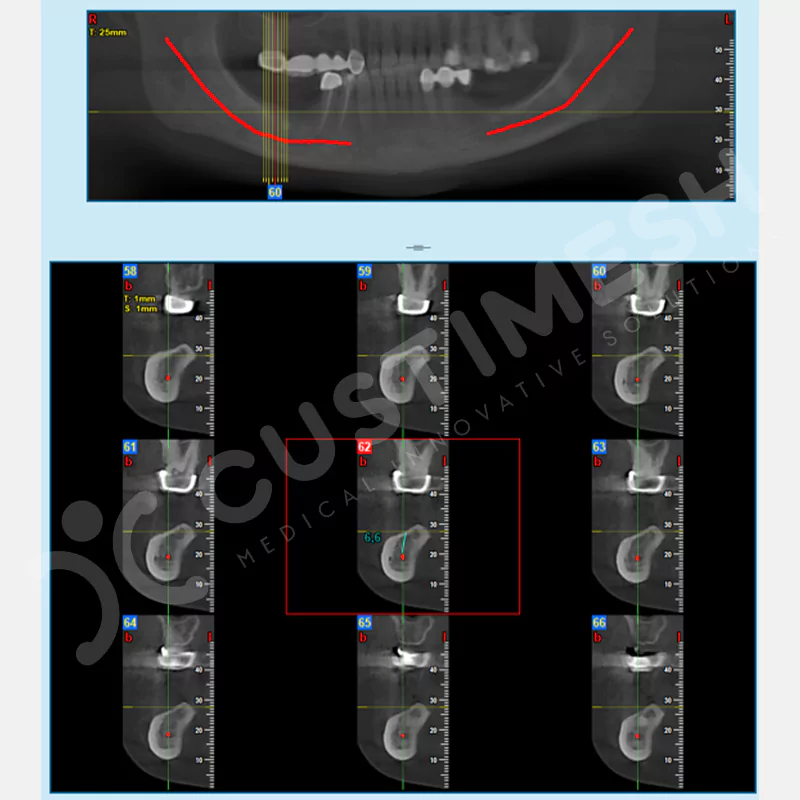
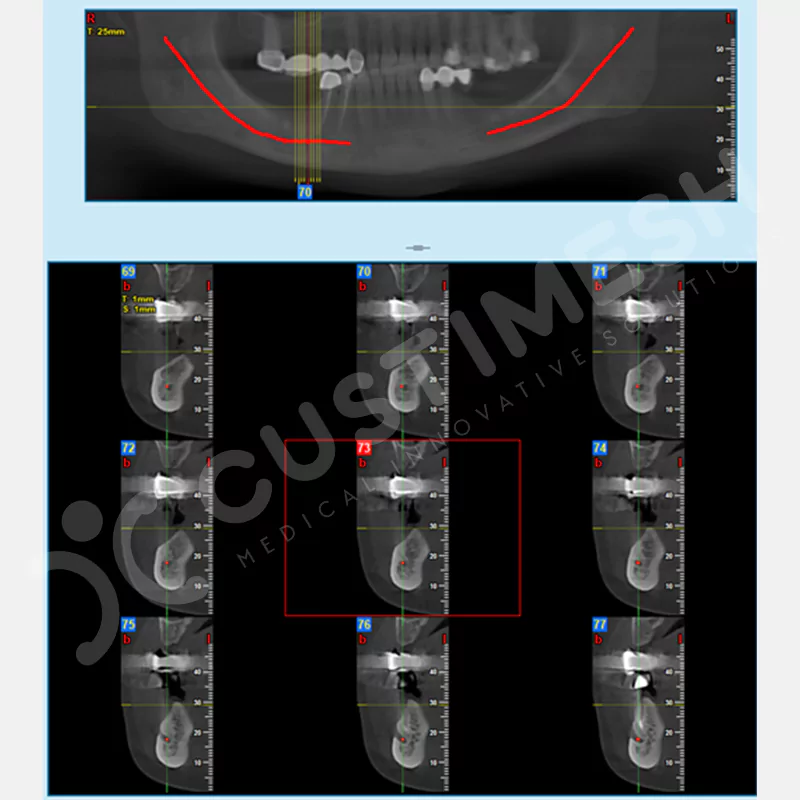
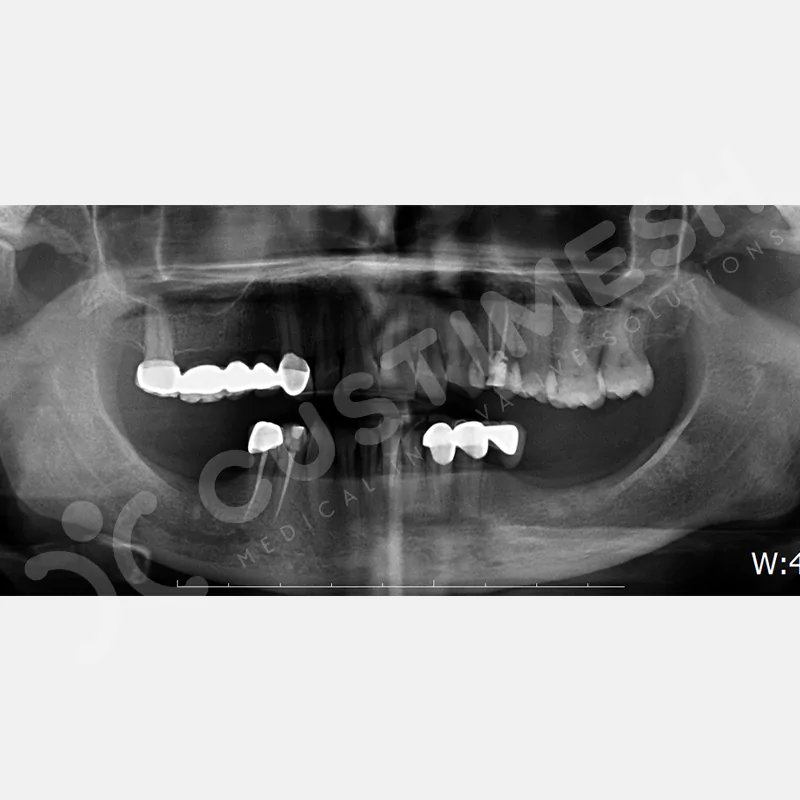
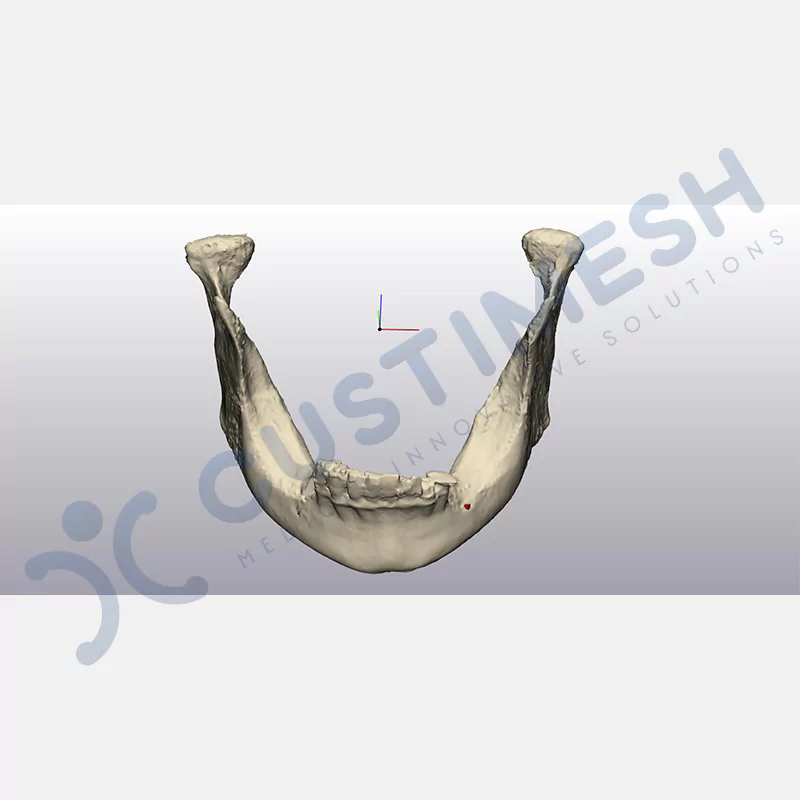
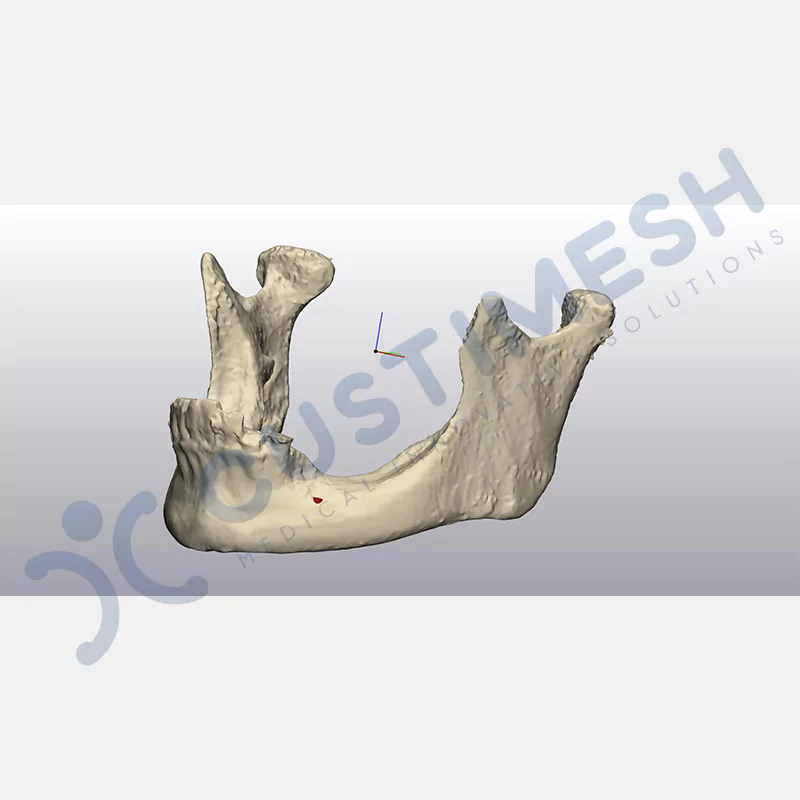
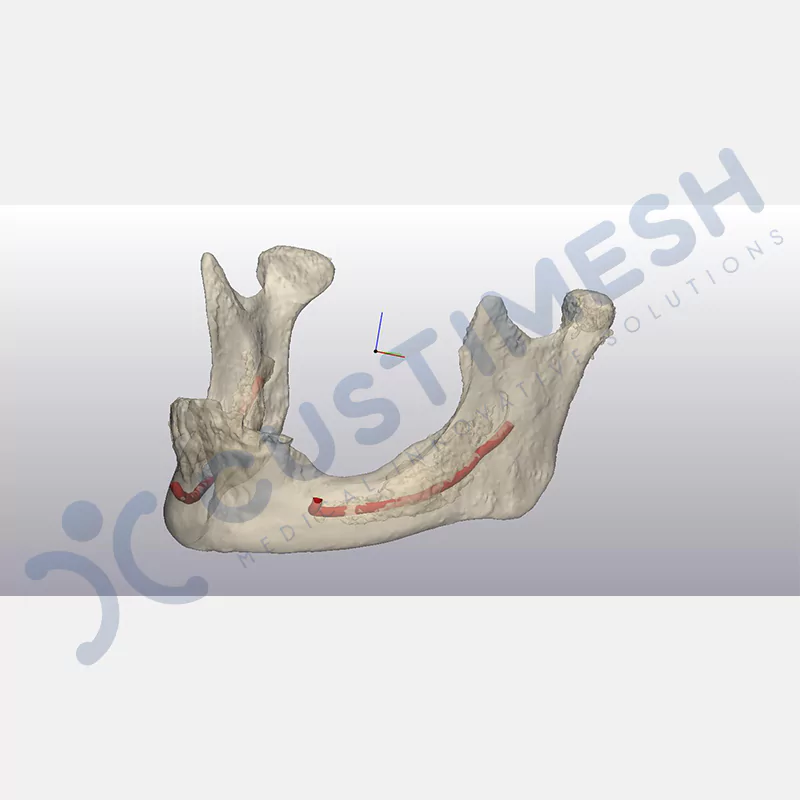
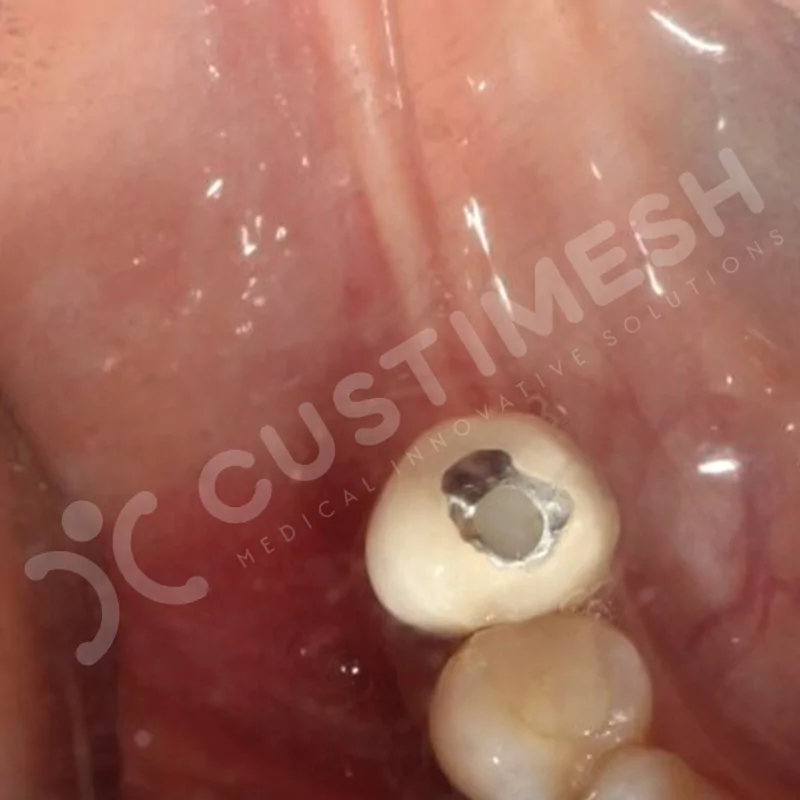
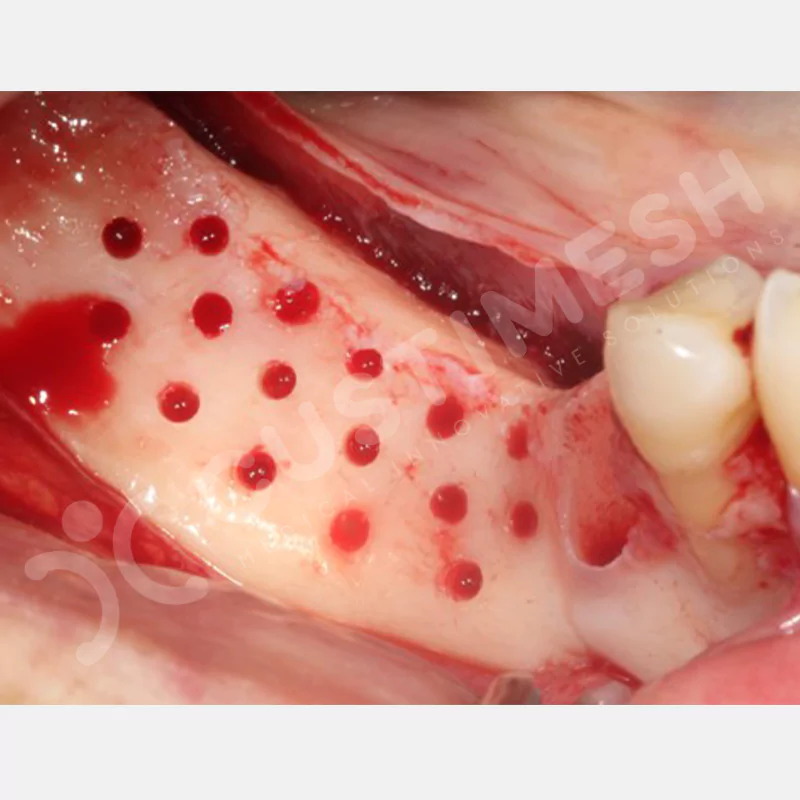
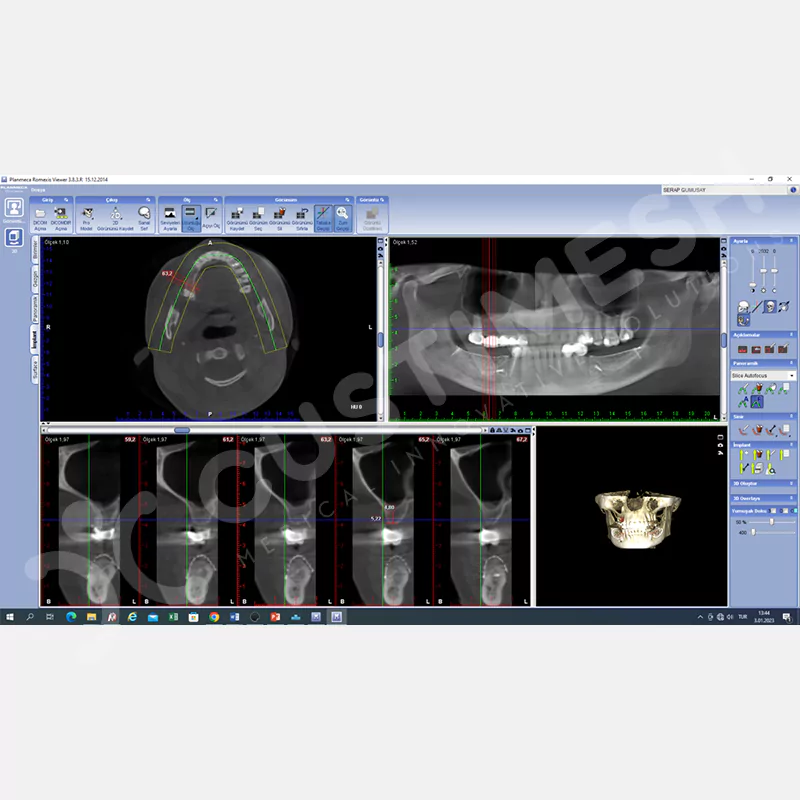
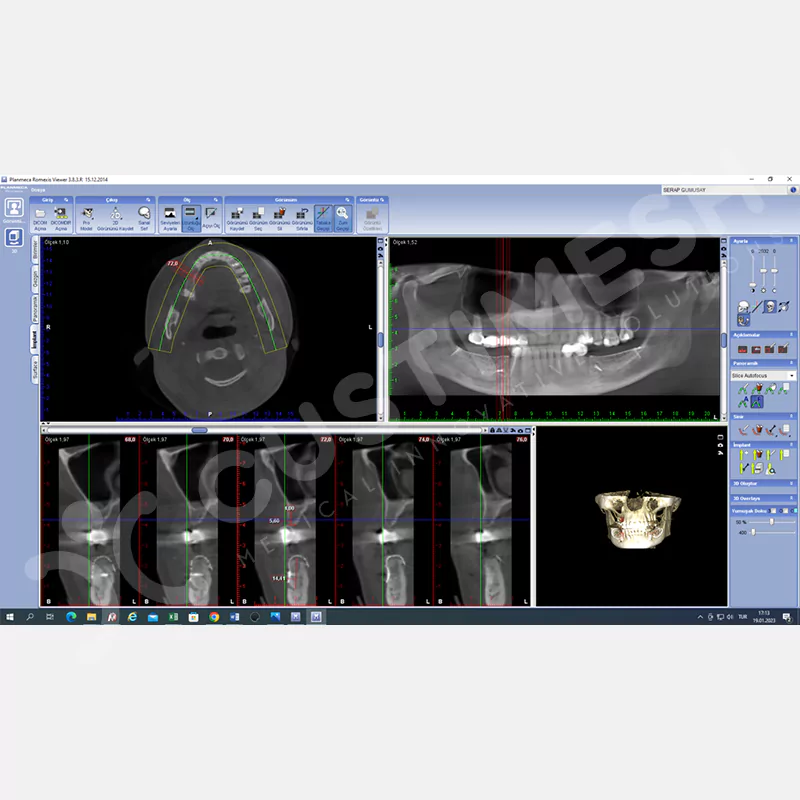
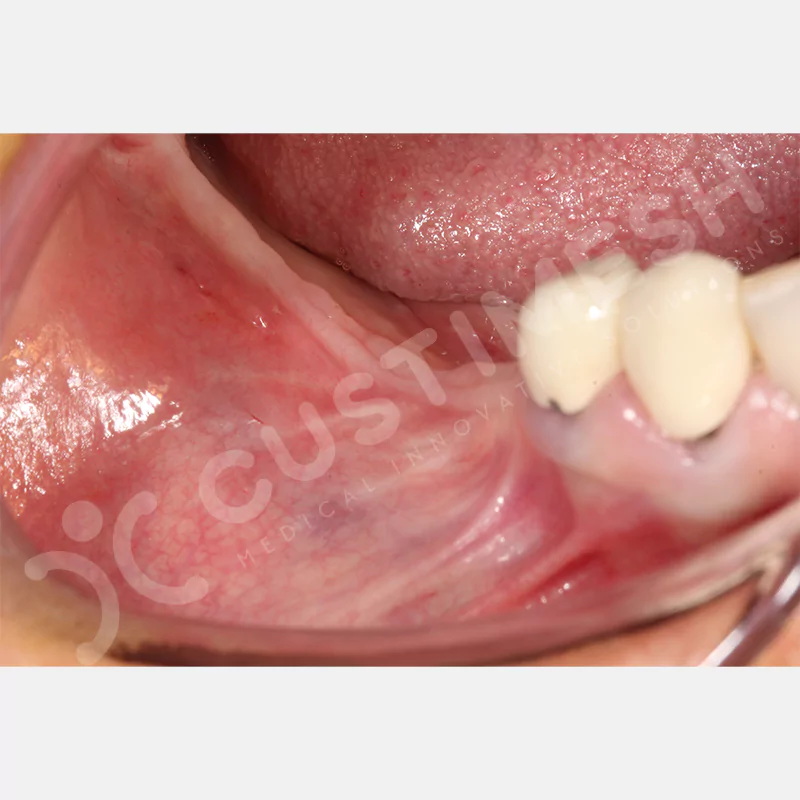
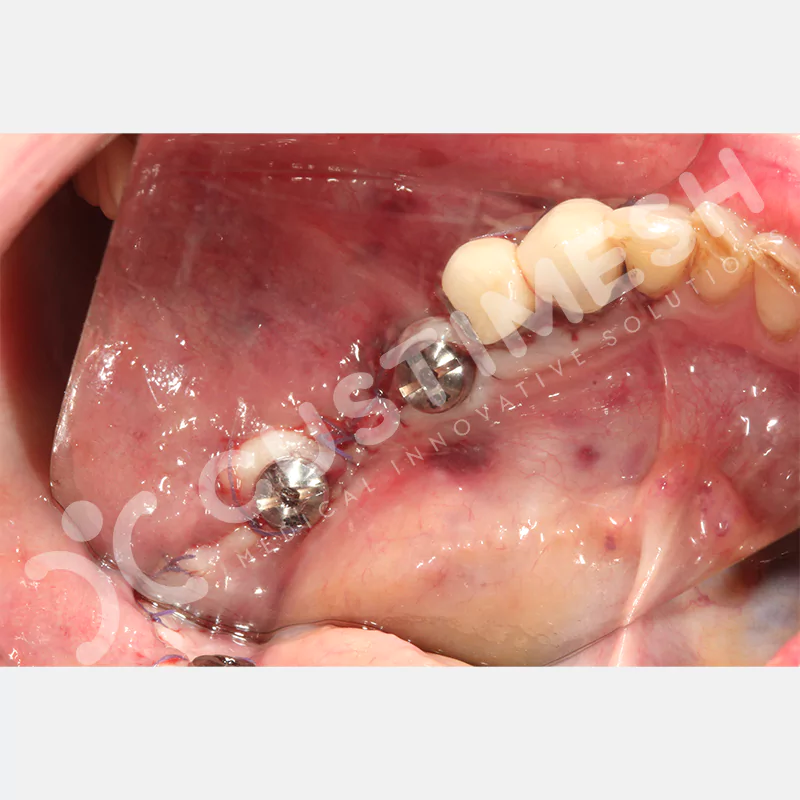
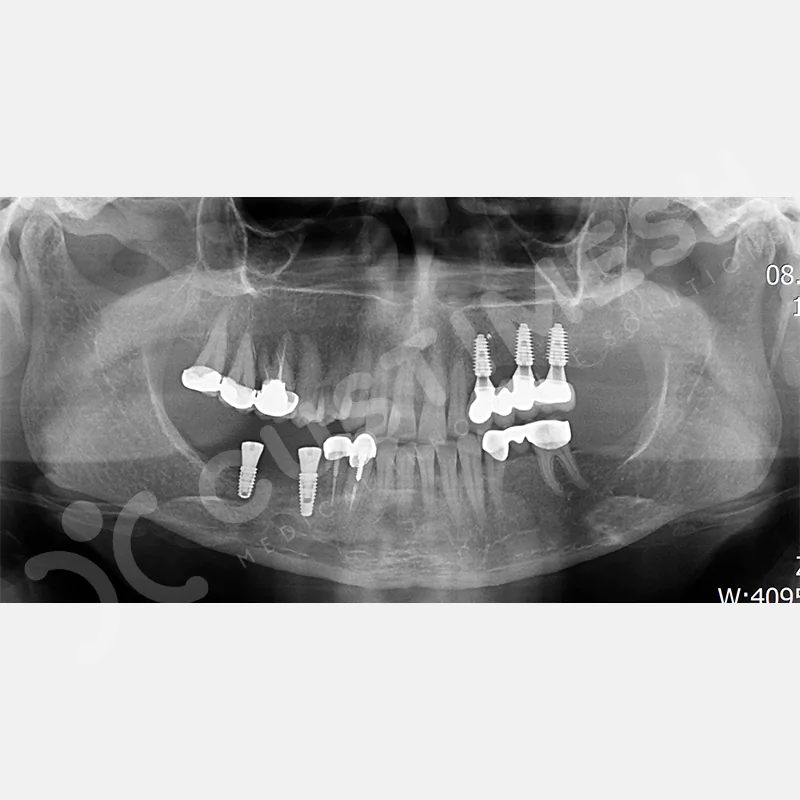
CASE 1
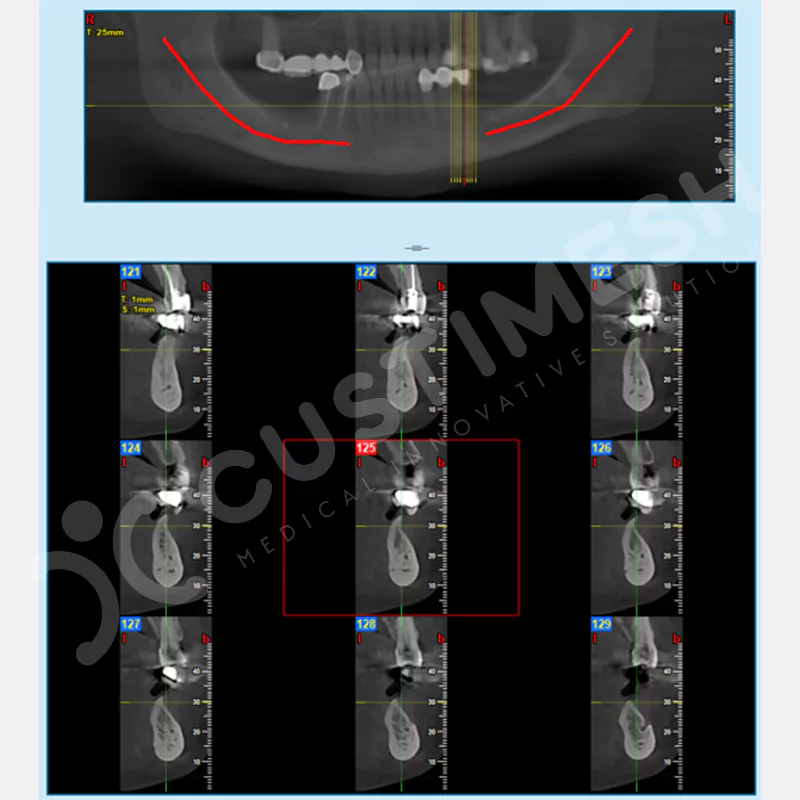
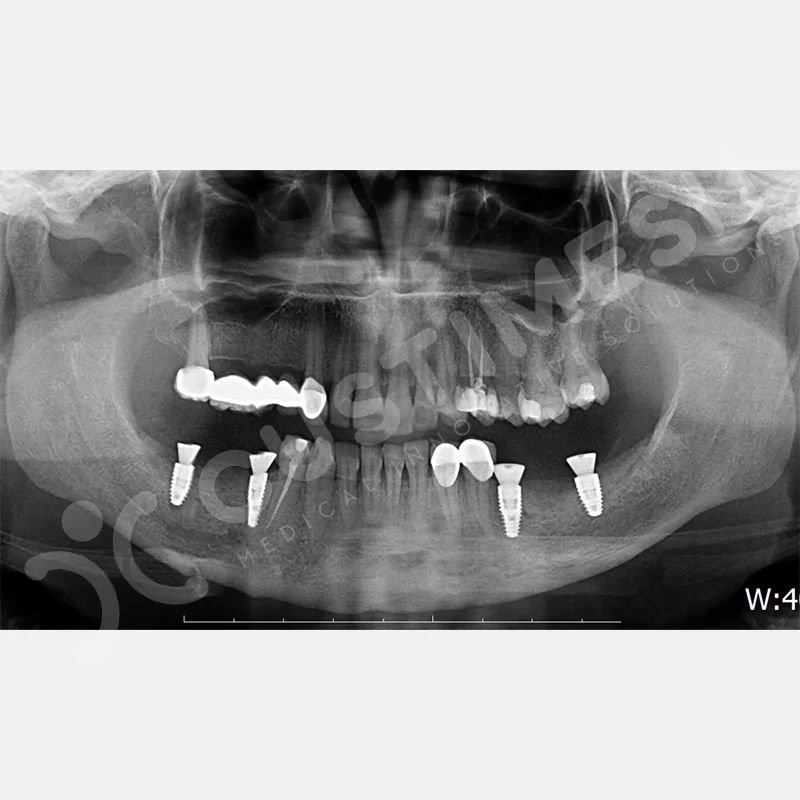
CASE 2
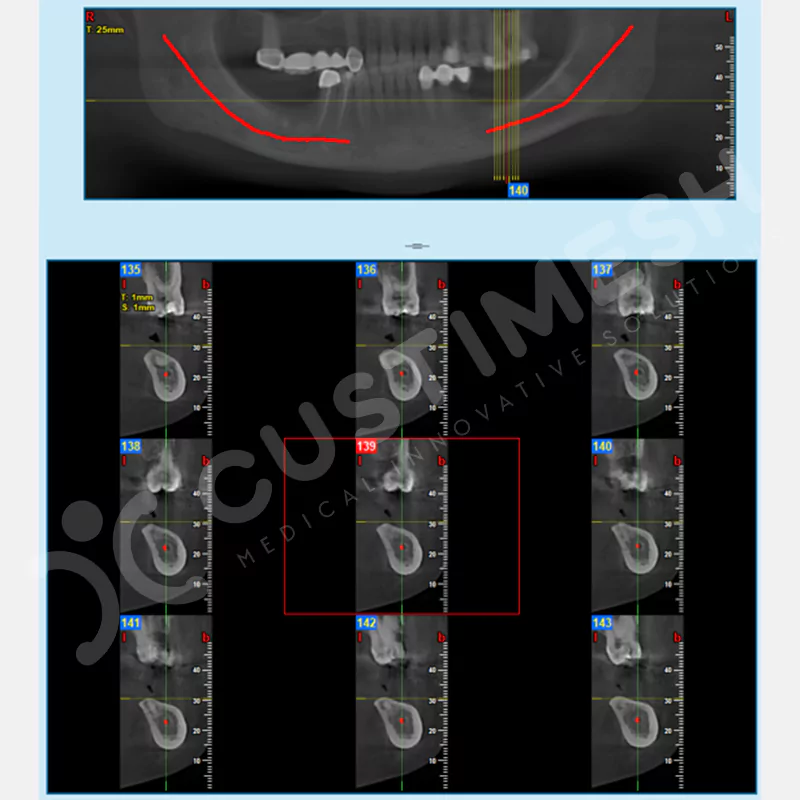
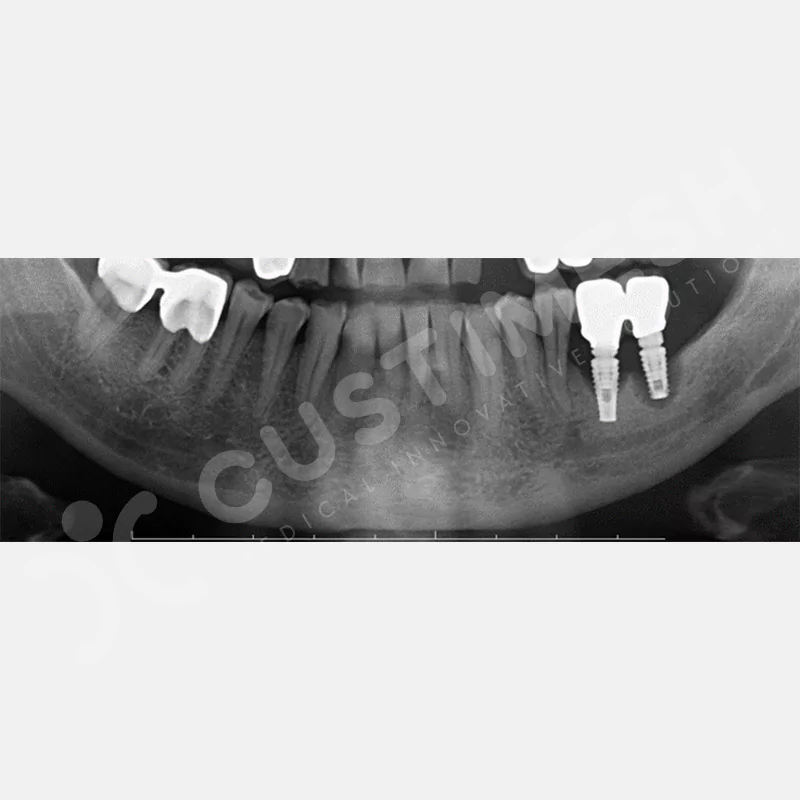
CASE 3
250+
210+
120+
Organisations we work with
Yıldırım Beyazıt Mah. Aşık Veysel Bulvarı No:63/T Erciyes Teknopark Melikgazi/Kayser Türkiye
Monday-Saturday 9:00 – 18:00