Prerequisites
Each Custimesh® product is intended for a specific patient with data provided by the doctor and may only be used for the intended patient. Particular caution is recommended in patients with autoimmune diseases, patients undergoing radiation or chemotherapy, heavy smoking habits and metabolic diseases that are usually uncontrolled. All precautions taken prior to surgical procedures should also be applied to patients who are taking bisphosphonates or being treated with RANKL antagonists or who have acute or chronic infections at the surgical site. In general, all preconditions to be considered for any bone augmentation also apply to Custimesh® patients.
Sterilisation
Custimesh® is supplied non-sterile in an autoclave pouch and must be sterilised before use on the patient. If the physician wishes, he/she can examine Custimesh® on the model without sterilisation before surgery. The product should then be repackaged in an autoclave bag and sterilised in an autoclave with steam under tension for 4 minutes at 132 degrees centigrade or 3 minutes at 135 degrees centigrade.
The sterilised titanium skeleton can be used directly for surgery.
Treatment Planning
If the patient has a horizontal and/or vertical bone defect, Custimesh® allows volume increase through bone regeneration. It stabilises the bone graft, allowing bone healing to take place undisturbed. Indications for the use of Custimesh® are horizontal and vertical or three-dimensional alveolar ridge augmentations.
Custimesh® is designed based on CBCT data using CAD-CAM programmes. The DICOM format file of the CBCT is used in Mimics® to create a 3-dimensional (3D) model of the area to be customised with titanium mesh and the model is transferred to 3-matic® for design. In order for the implant to be placed, a mesh structure is created by designing a surface perfectly compatible with the model, and this mesh structure exported in STL format is produced from titanium with a 3D metal printer with laser sintering technology.
Bone augmentation with Custimesh® should mainly be created with gold standard autogenous bone and bone graft materials with osteoconductive properties that prevent resorption. Autogenous bone can be harvested from intraoral sites such as the ramus, symphysis, tuber. Grafting can be performed with 1-stage or 2-stage augmentation depending on the defect.
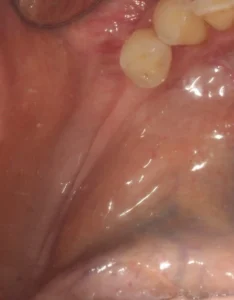
Soft tissue management is critical for the success of the treatment. Good release and tension-free closure of the flap is crucial. In general, the presence of a large adherent gingiva improves subsequent peri-implant hygiene and thus the long-term prognosis of implant-supported augmentation.
Furthermore, for the intraoperative use of Custimesh®, the patient’s oral hygiene must be very good from the preoperative period.
Surgical Technique
Incision
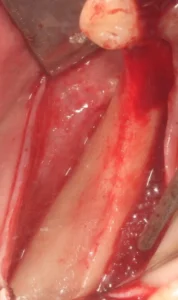
The incision to be made should be designed according to the anatomical structures such as maxillary sinus, nerves, vessels and ligaments according to the width and location of the bone augmentation area. A wide flap base is needed for adequate blood supply to the flap. The periodontal status of the neighbouring teeth should be taken into consideration as well as the prosthetic supports. The periosteum should not be damaged while lifting the flap and the periosteal incision for flap mobilisation should be made carefully so as not to disturb the nutrition of the flap.
Placing the Custimesh® Titanium Skeleton
1. In general, surgical procedures can be planned as careful preparation of the defect, bone harvesting for augmentation, placement of bone grafts into the defect area with Custimesh® and closure of the wound after three procedures:
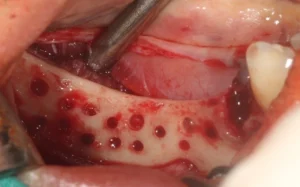
2. The next steps are lifting a mucoperiosteal flap, debridement of the granulation tissue and exposure of the defect. Decorticastone is recommended to improve blood supply to the defect area.
3. During placement and testing of Custimesh® in the defect site, a passive tension-free fit must be performed and biological conditions must be observed (maintaining a distance of 2 mm from neighbouring teeth or nerve structures).
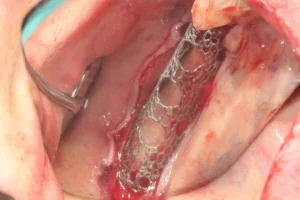
4. Bone is collected from donor sites in the mouth and the graft is prepared in particles. The mesh is then filled with autologous bone and bone graft. It should not be overfilled to avoid problems in fitting to the defect area.
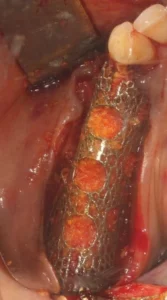
5. Titanium screws with a diameter of 1.4 mm are used for fixation of Custimesh®. The titanium screw must be inserted through the screw holes specified during design and in the lengths specified during design. The edges of the titanium scaffold must contact the underlying bone tissue. A resorbable membrane should be placed over the Custimesh® to prevent soft tissue from growing into the bone defect and to promote soft tissue regeneration on the titanium scaffold.
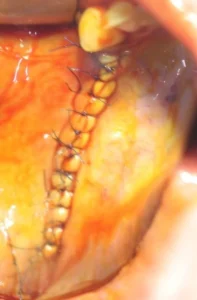
6. During wound closure, the mucoperiosteal flap is placed on the Custimesh® and sutured tension-free with simple, horizontal matrix sutures.
Wound Closure
Flep, mümkünse, titanyum iskelet üzerine gerilimsiz dikişlerle sıkıca dikilir (örneğin, basit ve horizontal matris dikişleri). Ameliyat alanında inflamatuar reaksiyon riskini azaltmak için yara tamamen kapatılmalıdır.
Custimesh®'in Çıkarılması
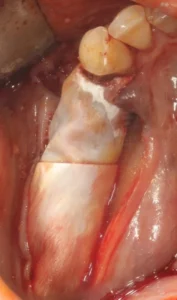
The surgical site should be checked regularly after suture removal and throughout wound healing for soft tissue condition, dehiscence and general signs of inflammation. After an uneventful bone healing phase (approximately 4-6 months), Custimesh® can be removed.
For removal of the Custimesh® and possible simultaneous placement of implants, the same (marginal) incision should be chosen as for grafting. After preparation of the mucoperiosteal flap, the mesh can be carefully removed after removal of the fixation screw. There is a splitting area on the Custimesh® for easy removal. Thanks to this area, the mesh can be mobilised with gentle lateral extrusion movements without excessive force, without affecting the augmentation. After removal of the Custimesh®, the implants can be placed in the new bone. If implant holes are prepared for implant placement in the Custimesh® design, it is possible to place implants without removing the mesh. The mesh can be removed after the implants have been placed.
Procedures in case Custimesh® is opened
According to the literature, even if the surgical procedure is uneventful, there is a 25% chance of dehiscence. The literature also shows that angulation does not affect the success of bone augmentation. It is important in which period of healing the dehiscence occurs and whether there are signs of infection with dehiscence. Openings seen in the early wound healing period may adversely affect the prognosis and are usually accompanied by infection. However, in openings occurring after the early period, it has been shown that it is sufficient to keep the area clean through regular washing by the patient and to be followed up by the physician with frequent controls.